
Attention deficit hyperactivity disorder (ADHD) is the most prevalent behavioral disorder in children, which has been estimated to affect 4-5 million children within the United States.1 Pharmacological and behavioral interventions have been the primary means of medical management of ADHD. However, the former approach has induced side-effects amongst approximately 65-70% of patients, and as many as 30% of said population are non-responders to drug therapies.1(533) The precise cause of ADHD is unknown but it thought to be multifactorial in nature.1(533) As such, the following will consider some biomarkers that may help direct nutritional and lifestyle changes to better manage ADHD.
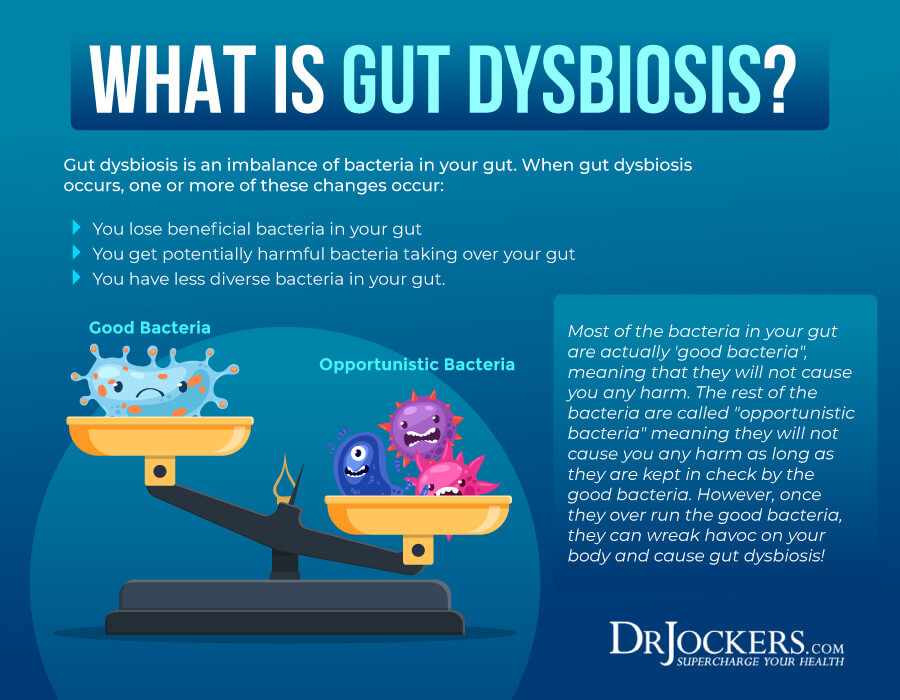
If multiple blood/organic acids (OAs) markers are out of range to include those of the gut microbiome, one approach could be addressing gut function/dysbiosis first. Gut dysbiosis is a condition characterized by a disturbance in the number and ratio of microorganisms (known as the microbiome) in the small and large intestine to include parasites, bacteria, fungus, and viruses and fungus.2 Said aberrations in gut function are linked to altered nutrient digestion/absorption, reduced immune/barrier function (which can be confirmed by high fecal SLGA levels), and altered behavioral (i.e., anxiety/depression) and cognitive function, such as ADHD.1(543)3,4,5 Thus, if gut function is sub-optimal, said condition is likely to produce unfavorable downstream systemic effects within the individual.

OA markers which could indicate dysbiosis would be high benzoate; a marker of intestinal bacterial overgrowth, which could emanate from celiac disease or short bowel syndrome.5(375) Thus, if such a marker is high, this author might suggest additional tests to determine the presence of said conditions. If another OA, hippurate, is simultaneously high (formed from benzoate conjugating with glycine) such markers also help confirm liver detoxification challenges (phase 2 glycine conjugation).5(376) Furthermore, if other detoxification markers are high (i.e., pyroglutamate; a marker of glutathione demand), such can help further confirm detoxification issues.5(367) Phenylacetate (PAA) is another OA which helps assess gut function. Intestinal bacteria raise PAA levels when they interact with dietary polyphenols (from plant food).5(376) Ultimately, individuals with normal intestinal function will have only background levels of PAA in the urine.5(376)
High OA levels of p-hydroxybenzoate (PHB) are formed from bacterial action on dietary proteins and polyphenols.5(378) Furthermore, strains of Escherichia coli (E. coli) can produce high levels of PHB indicating a potential overgrowth of said bacterial strain.5(378) Indican is another OA which, when at higher levels, suggests bacterial overgrowth in the upper bowel. Furthermore, individuals suspected of having celiac disease are at greater risk of higher readings of indican because their condition allows for undigested proteins (poor digestion is a characteristic of celiac disease) containing tryptophan to be acted upon by such bacterial strains.5(384)

Another OA, D-arabinitol, is also high when there is an overgrowth of fungus (Candida) in the gut.5(387) Such can occur from antibiotic use, as said medication tends to kill bacteria allowing for an overgrowth of fungus measured by high D-arabinitol levels. Thus, this author would ask clients with ADHD if they used a course of antibiotics recently as a means of determining the nature of said overgrowth. Candida species also tend to proliferate in the presence of carbohydrate sources.6 Thus, ingestion of said macronutrient (while measuring D-arabinitol) is likely to increase the presence and proliferation of Candida. Consideration of fungal overgrowth is essential as it can induce bloating, discomfort, and diarrhea, which can lead to nutrient deficiencies.7
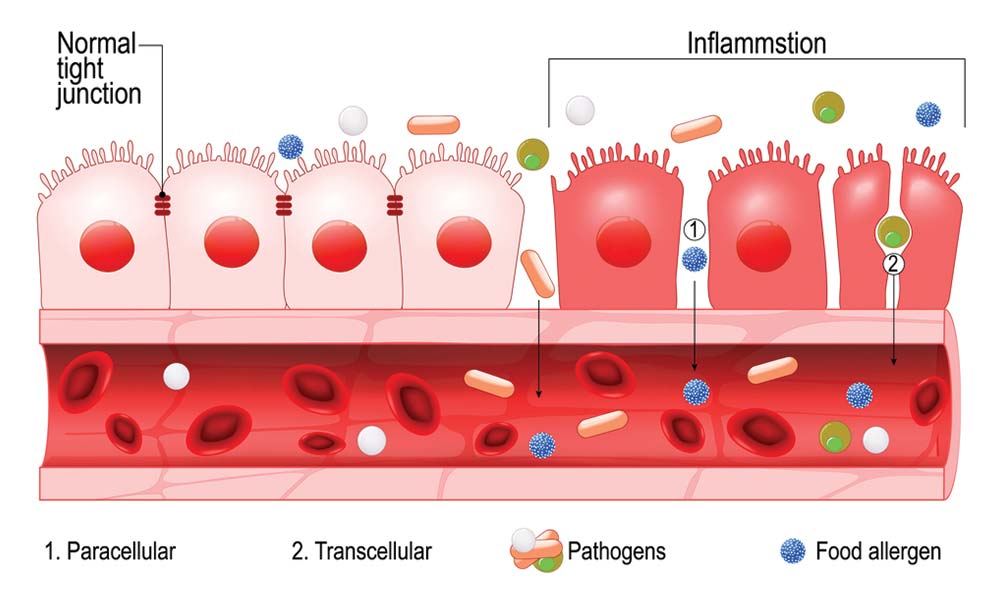
Having considered markers of gut dysbiosis (benzoate, hippurate, phenylacetate, p-hydroxybenzoate, indican, D-arabinitol) as well as inflammatory/compromised gut barrier function (SLGA) markers and detoxification status (pyroglutamate), it is imperative to develop nutritional strategies to help return said biomarkers back to normal ranges. If food antibody (measuring IgG4 antibody) tests are conducted (i.e., dairy, meat, poultry, fish, legumes, fruits, grains, vegetables, nuts, seeds), removal of foods associated with severe responses is a logical first step. Such is a prudent measure as IgG4 antibodies are clinically relevant markers of food-immune reactions and possible causes of intestinal permeability.5(435)
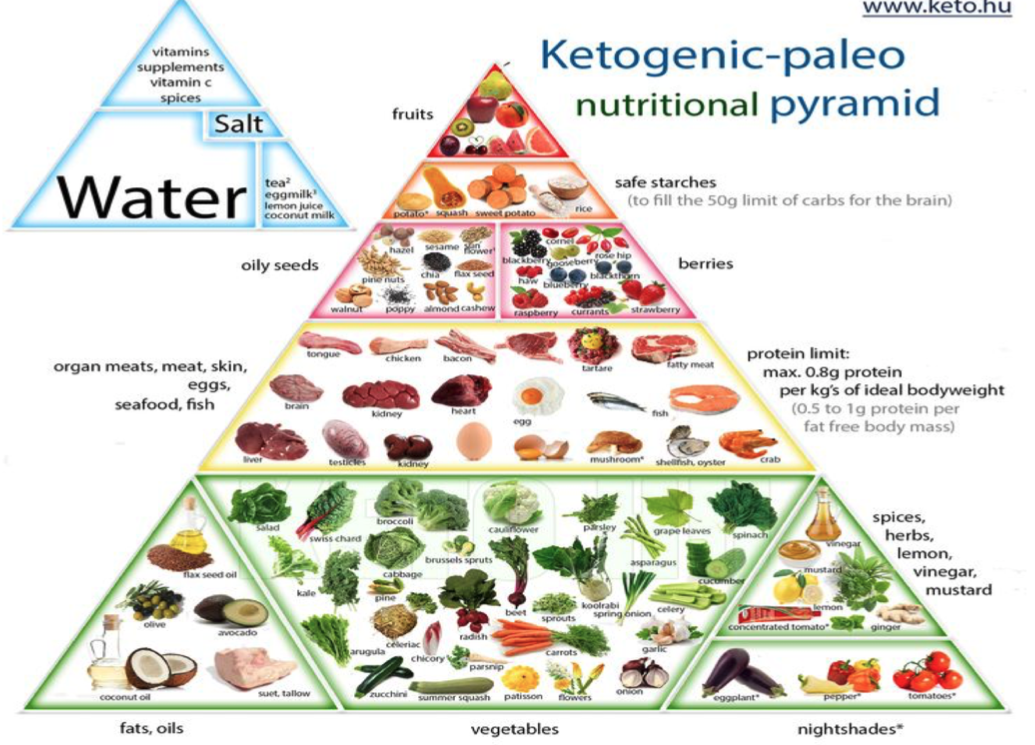
Reduction of processed carbohydrates would also help restore optimal gut function.6(198) Since D-arabinitol levels/fungal overgrowth, as indicated in previous sections, proliferate in carbohydrate-rich environments, removal of foods (i.e., table sugar, cereals) would help starve out Candida and likely facilitate reduction of bloating and diarrhea, if present. Other options to help restore gut function and digestion, according to Lord et al5(453) would include nutritional support implementing vitamins A, C, B1-B12, selenium, chromium, molybdenum, manganese, magnesium (which could come from a multivitamin) and amino acids such as glutamine (a primary energy source for intestinal tissue) and glutathione (a powerful antioxidant).

Implementation of a probiotic foods/supplements with organisms favourable to the gut and the use of foods to support their growth (i.e., pre-biotic) in the form of dietary fiber (raw/cooked vegetables) would help repopulate favorable species of bacteria.5(390)Examples of beneficial bacterial strains could include L. acidophilus, L. sporogenes, Bifidobacteria sp., S. boulardii, and soil organisms.5(390) Improving liver detoxification (as measured by high pyroglutamate/benzoate/hippurate) could be achieved by introducing nutrients to boost glutathione production to include N-acetylcysteine (NAC), in addition to increasing antioxidants mentioned in the previous section.5(368)
In conclusion, ADHD is the most prevalent behavioral disorder in children, which has been estimated to affect 4-5 million children within the United States. Pharmacological and behavioral interventions have been the primary means of medical management of ADHD with varying degrees of efficacy. As an adjunct therapy, nutritional support via removal of antagonizing foods and introduction of other foods/supplements to support proper immune function and digestion would be feasible and low-risk approaches to attenuate gut dysbiosis, if present. Though the aforementioned sections provide a degree of insight and solutions to improving gut/immune/detoxification health, it is by no means a complete intervention in helping manage ADHD. However, implementing such approaches would be another vital step for improving ADHD-related symptoms and overall quality of life.
References
1. Kohlstadt I. Advancing Medicine with Food and Nutrients. 2nd ed. London, NY: CRC Press; 2012.
2. Huang L, Gao R, Yu N, et al. Dysbiosis of gut microbiota was closely associated with psoriasis. Sci China Life Sci. 2019;62(6):807-815. doi:10.1007/s11427-018-9376-6.
3. Cao H, Liu X, An Y, et al. Dysbiosis contributes to chronic constipation development via regulation of serotonin transporter in the intestine. Sci Rep. 2017;7(1):1-12. doi:10.1038/s41598-017-10835-8
4. Zu X, Wang C, Dong X, et al. Chronic alcohol exposure induced gut microbiota dysbiosis and its correlations with neuropsychic behaviors and brain BDNF/Gabra1 changes in mice.Biofactors. 2018;45(2):187-199. doi:10.1002/biof.1469
5. Lord RS, Bralley, JA. Laboratory Evaluations for Integrative and Functional Medicine. 2nded. Duluth, GA: Genova Diagnostics; 2012.
6. Jeziorek M, Frej-Mądrzak M, Choroszy-Król I. The influence of diet on gastrointestinalcandidaspp. colonization and the susceptibility of candida spp. to antifungal drugs. Rocz Panstw Zakl Hig. 2019;70(2):195-200. doi: 10.32394/rpzh.2019.0070.
7. Erdogan A, Rao SSC. Small intestinal fungal overgrowth. Curr Gastroenterol Rep. 2015; 17(4):1-7. doi:10.1007/s11894-015-0436-2.
-Michael McIsaac
