Alzheimer’s disease (AD) is a condition which has increased its reach exponentially, and worldwide, over the last 10 years.1 AD is characterized by the development of two abnormal proteins in the brain known as tau and β-amyloid, which are toxic to nerve cells within the brain.2 Aggregation of said proteins inexorably leads to neuron destruction, degraded brain function, and manifestations of dementia; a term, which describes a deterioration in mental abilities including memory, logical thinking, and language.2(1488) As such, it is imperative to employ interventions that help slow said degenerative processes. The following will consider AD in greater detail, and nutritional protocols to help support health and control the pathophysiology of AD.
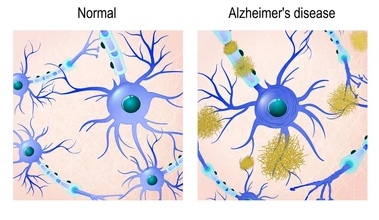
In a normal physiological state, there is a harmony and balance between production and degradation of excess β-amyloidand tau levels.1(737) However, during the disease progression, an aberration occurs between production and clearance of such proteins, which leads to protein aggregation, neuroinflammation, and neurodegeneration.1(737) Interestingly, individuals with metabolic disorders such as hypertension, obesity, and diabetes mellitus have a higher risk of AD. Thus, specific biomarkers from patients with metabolic disorders that can detect or prevent AD progression at earlier stage of its development is critical.1(737)
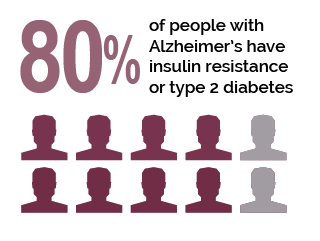
Jayaraj et al1(737) indicated that several studies supported a relationship between insulin resistance (IR) and impaired insulin signaling; such conditions are considered dominant pathological factors in both diabetes mellitus (DM) and AD.1(737) During IR, as discussed in previous posts, higher than normal concentrations of insulin are secreted from the pancreas to clear glucose from the bloodstream. Such a scenario is also known as impaired insulin sensitivity.3 Furthermore, insulin is also known to regulate synaptic and neuronal function within the cortex, cerebellum and hippocampus and protect neurons from cell death.1(741)
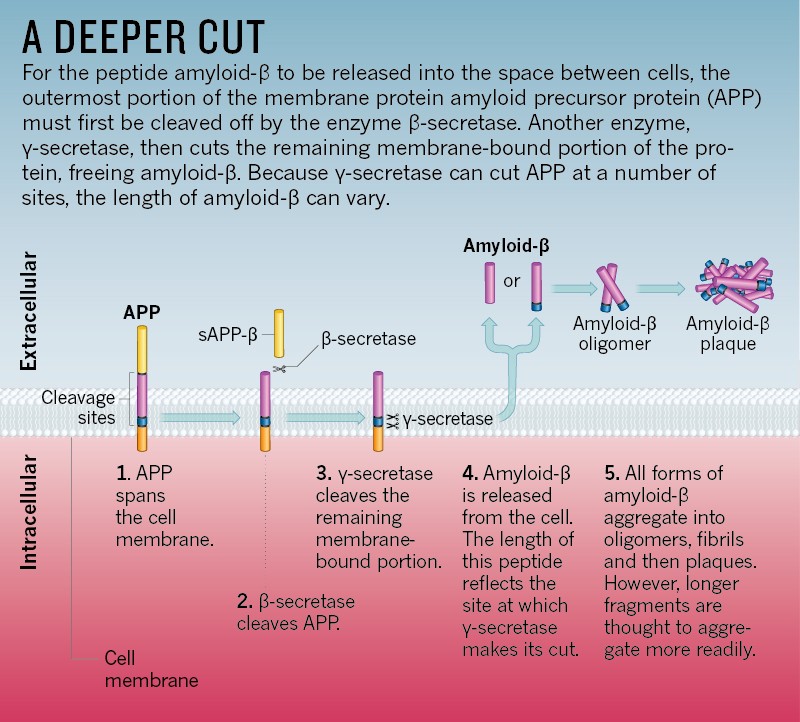
Insulin achieves such successful regulation by interfacing with β-amyloid precursor protein cleaving enzyme 1 (BACE1) and gamma-secretase to regulate β-amyloid levels, which degrades excess β-amyloid by modulating insulin-degrading enzyme (IDE).1(741) Thus, insulin signaling impairment (aka IR) in type 2 diabetes (T2D) and AD have a direct effect on the buildup of β-amyloid levels.1(741) Similar issues arise from IR and tau; IR/deficiency can induce aberrant activation of glycogen synthase kinase-3b (GSK3B) and hyperphosphorylation of tau in both T1DM and T2DM.1(741) GSK3B is an enzyme that when activated, leads to tau formation/neurofibrillary tangles; a primary marker of AD.1(741) Having briefly considered the pathophysiology of AD and its relationship to IR, the following will explore solutions for the same.
Although AD is multifactorial, managing risk factors mentioned previously (obesity, IR, hypertension) is a logical first step. As such, annual screening for diabetes, hypertension, and other diseases to include as cancer and heart disease could help facilitate early detection and treatment.4 If IR is detected (i.e., via fasting glucose/fasting insulin, post-prandial glucose/post-prandial insulin), one method to improve insulin sensitivity and mitigate hyperinsulinemia is through low carbohydrate diets.5 Such dietary interventions have been shown to normalize blood glucose levels, reduce bodyfat, decrease hunger, improve blood lipid profiles, as well as reducing the need for medications.5(1-9)
It is this author’s opinion that reducing overall refined carbohydrate load would provide the most meaningful and widespread benefit to individuals at risk for AD, especially if they are diagnosed with IR. However, considerations do exist beyond simply controlling IR; other nutritional interventions to contemplate would the use of omega-3 fatty acids (O3FAs).4(496) Such is the case since O3FAs contain substances which have neuroprotective properties and can have beneficial effects on managing neurodegenerative processes.4(496) Kohlstadt4(496) stated that reduced levels of said fatty acids was associated with age-related cognitive decline. Conversely, the use of O3FAs exhibited the ability to down-regulate excitotoxic glutamate neurotransmission, arachadonic acid dominance, and amyloid oligomer prevalence.4(496)
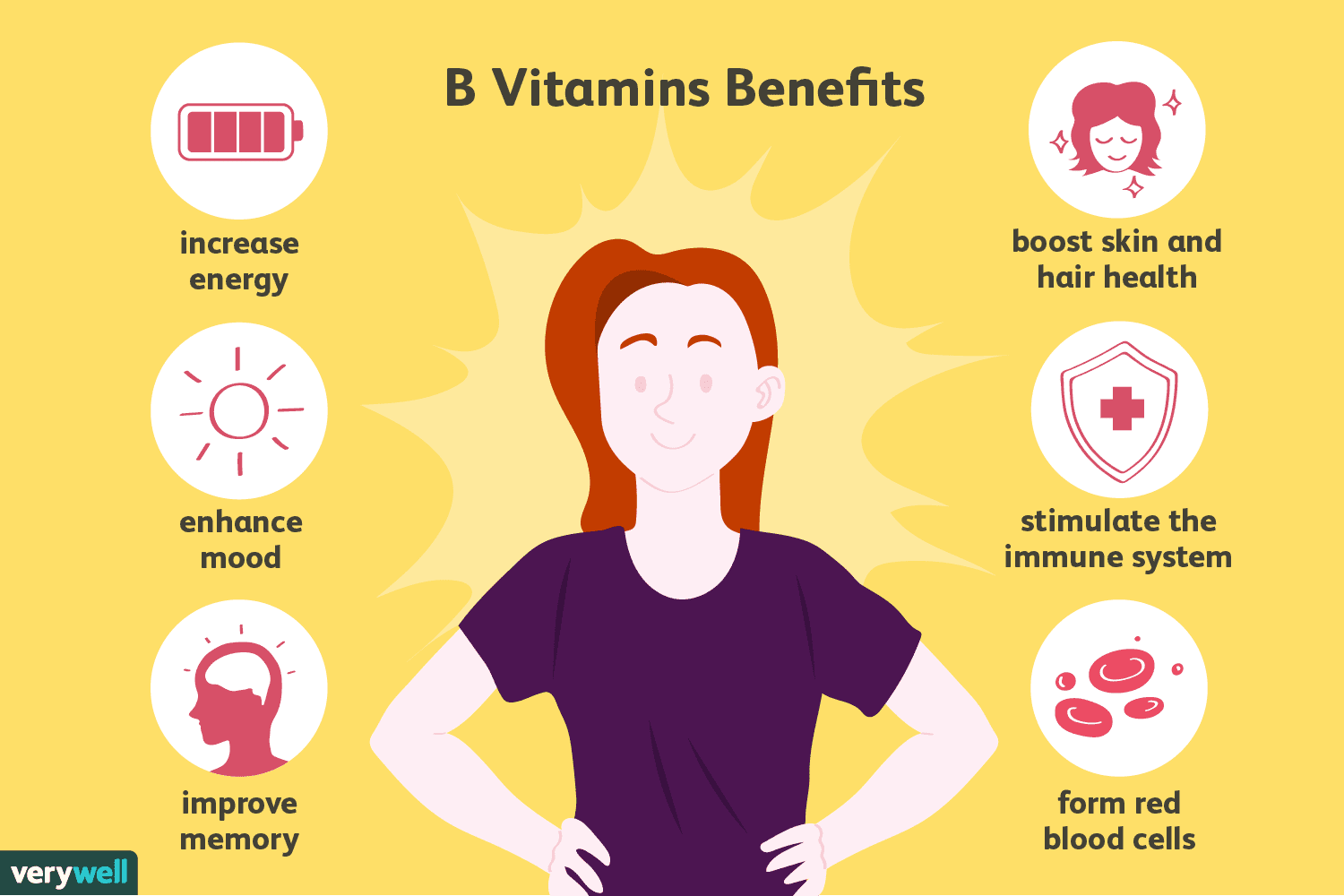
B-vitamins, or lack thereof, have also been implicated in cognitive decline, especially if homocysteine levels (a marker of oxidative stress) are above normal ranges (above 11.4 umol/L).4(497) Kohlstadt4(497) cited a double-blind placebo-controlled study whereby individuals in the test group with mild cognitive impairment (MCI) were supplemented with folic acid (B9), B12, and B6. Results indicated that, when compared to baseline measures, homocysteine levels returned to normal ranges and global cognition, semantic memory, and episodic memory improved.4(497)

Vitamin D levels (measured as 25-hydroxyvitamin D or 25OHD) is another metric to consider for those at risk for AD. Optimal concentrations of 25OHD, in the brain, suggests a potential neuroprotective effect to include regulation and management of calcium homeostasis. Such is achieved by down-regulating calcium channel expression along neuronal cell membranes.4(497) Furthermore, additional evidence supports that sub-optimal levels of 25OHD (less than 10 ng/ml) has been strongly associated with considerable cognitive decline.4(497)

In conclusion, AD is a condition which has increased its reach exponentially, and worldwide, over the last 10 years. Such a condition is characterized by neuron destruction, degraded brain function, and manifestations of dementia; a term, which describes a deterioration in mental abilities including memory, logical thinking, and language. Despite the deleterious effects of AD, early detection by monitoring the aforementioned biomarkers can serve as a critical first step in treating the disease, and its progression. Close consideration of particular nutrients to include B vitamins, O3FAs, and vitamin D can also help slow the development of AD in addition to reducing overall refined carbohydrate load. As an aggregate, said protocols can provide at-risk individuals the opportunity to use nutrition, as medicine; an approach that can help support cognition, health, and overall quality of life.
References
1. Jayaraj RL, Azimullah S, Beiram R. Diabetes as a risk factor for Alzheimer’s disease in the Middle East and its shared pathological mediators. Saudi J Biol Sci. 2020;17(2):736-750. doi: https://doi.org/10.1016/j.sjbs.2019.12.028
2. Jin J. Alzheimer disease. JAMA. 2015;313(14):1488. doi:10.1001/jama.2015.2852.
3. Lord RS, Bralley, JA. Laboratory Evaluations for Integrative and Functional Medicine.2nd ed. Duluth, GA: Genova Diagnostics; 2012; 20:317-331.
4. Kohlstadt I. Advancing Medicine with Food and Nutrients. 2nd ed. London, NY: CRC Press; 2012.
5. Feinman RD, Pogozelski WK, Astrup A, et al. Dietary carbohydrate restriction as the first approach in diabetes management: Critical review and evidence base. Nutrition. 2015;31(1):1-13. doi:10.1016/j.nut.2014.06.011.
-Michael McIsaac
