Arterial hypertension (AH) is considered the predominant risk factor for cardiovascular disease (CVD), and is defined as a resting systolic blood pressure of ≥140 mmHg and/or diastolic blood pressure of ≥90 mmHg in adults.1 According to Lopes et al,1(65) the number of individuals with hypertension has increased in a precipitous fashion since 1980, worldwide. Considering that AH is associated with an increased risk of myocardial infarction, congestive heart failure, kidney disease, and stroke, it is imperative to implement strategies which support optimal blood pressure ranges.1(66) Occasionally, when nutrition and lifestyle changes fail to lower blood pressure, medications are indicated. However, research has suggested that drug-nutrient interactions exist which can induce micronutrient deficiencies and secondary health issues. As such the following will explore the same in greater detail.
Mohn et al2 stated that long-term use of both prescription and over-the-counter drugs can induce subclinical and clinically relevant micronutrient deficiencies. Said deficiencies can slowly develop, in an almost insidious fashion, over months or years allowing aberrations in health to slowly manifest, unnoticed.2(1) Furthermore, nutrient deficiencies often do not present as classically described, and many health care providers are not trained to detect the same and often attribute deficiencies to a disease instead.2(1)
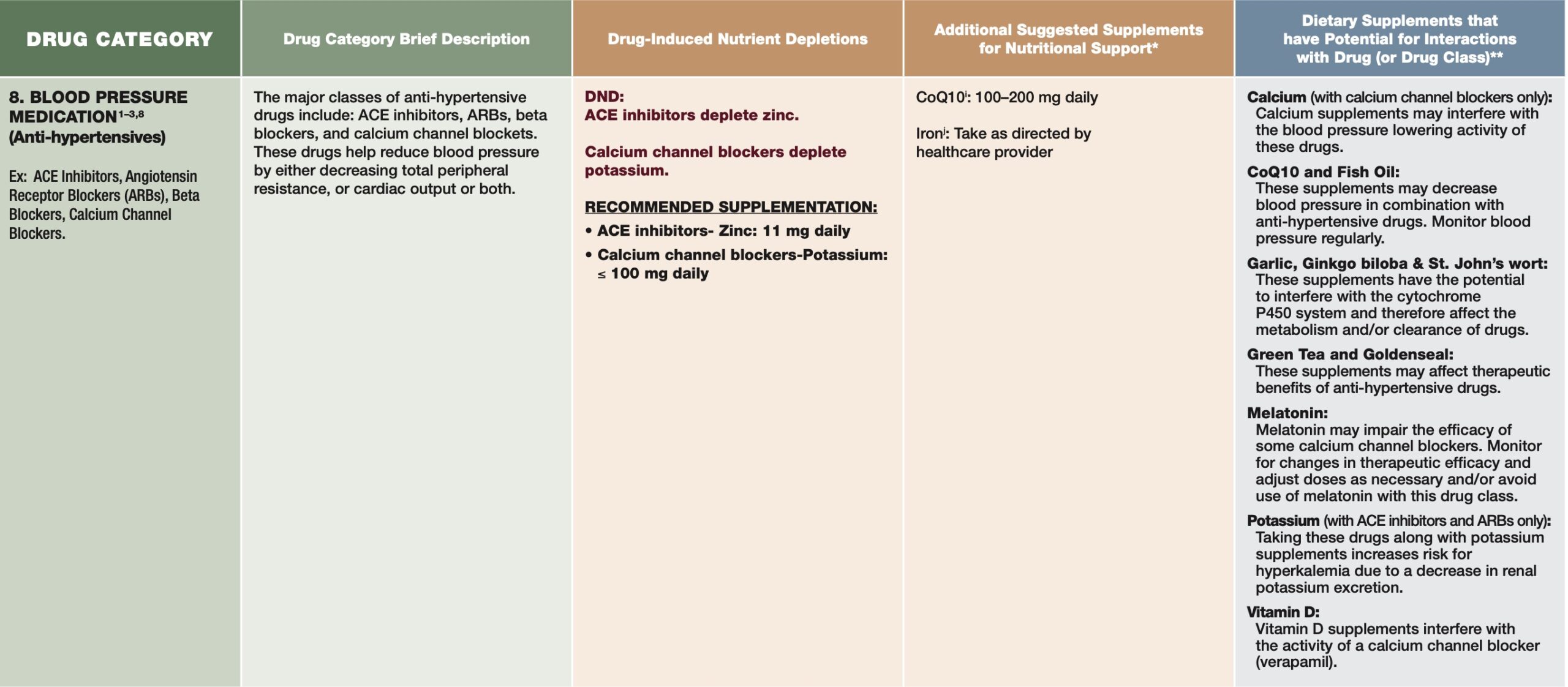
Such drug-induced interactions, defined as physical/physiological/chemical/pathophysiological relationships between a drug and nutrient/s, can negatively influence digestion, consumption, absorption, distribution, and metabolism of said nutrients.2(1) As such, knowledge of micronutrient deficiency trends related to certain drugs, and tracking of biomarkers, is critical. The following will consider anti-hypertensive drugs (AHDs) and associated nutrient interactions in greater detail.
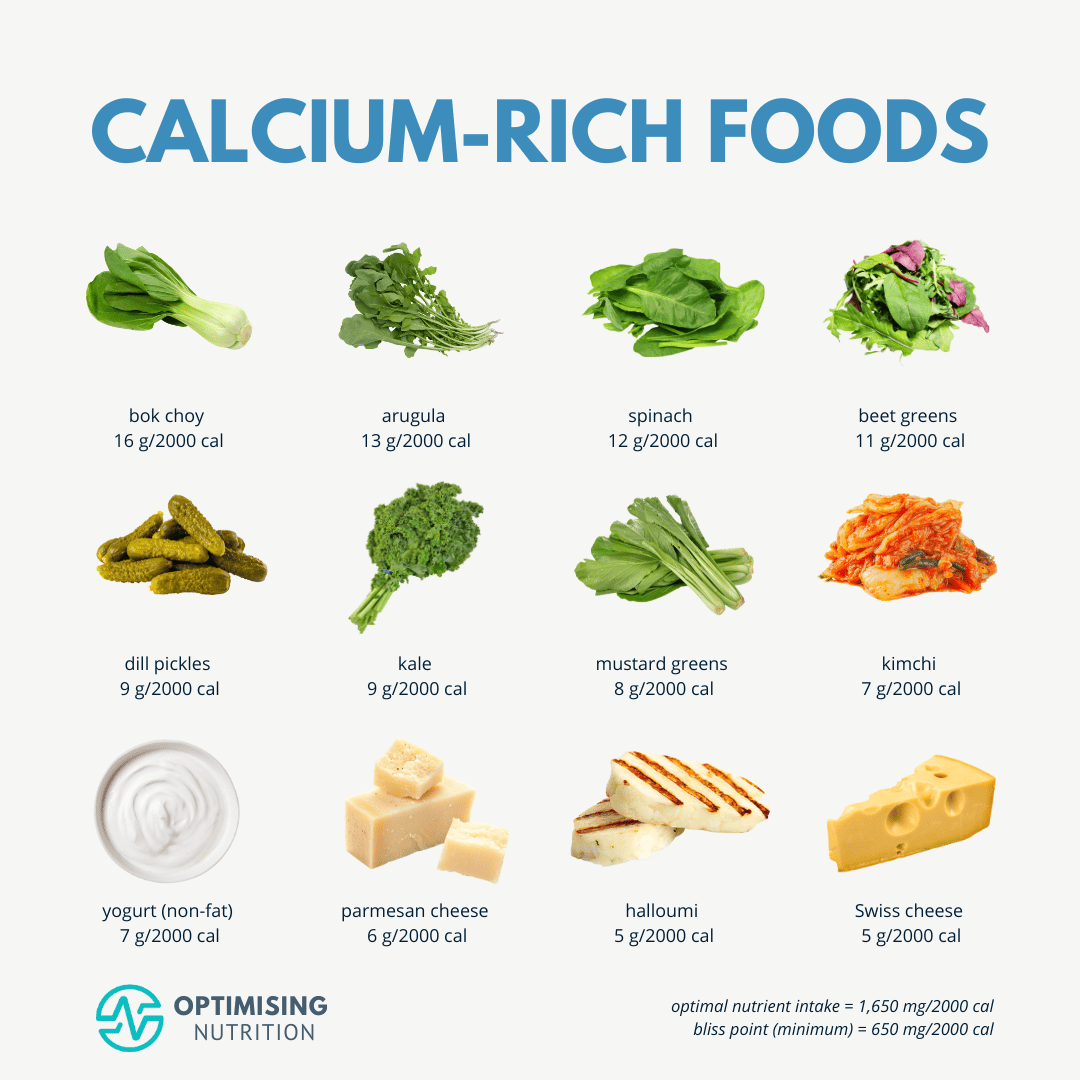
AHDs such as calcium and loop diuretics can increase calcium excretion through the kidney in addition to increasing parathyroid hormone (aka PTH which increases osteoclast/bone breakdown).2(9) Left unmitigated, heightened calcium loss and elevated PTH synthesis can induce losses in none mineral density and increased risk of fractures.2(10) Ultimately, such findings suggest the potential of loop diuretics negatively influencing calcium homeostasis and a possible cause of secondary hyperparathyroidism.2(9)

AHDs can also affect magnesium status amongst users of loop and thiazide diuretics.2(11) Specifically, magnesium reabsorption within the kidney can become compromised both from short-term and long-term use of loop diuretics, while thiazides indirectly reduce magnesium by supressing PTH synthesis and release.2(11) Of particular note; diuretic therapies modestly lower serum magnesium levels but significantly lower cellular magnesium concentrations, which could lead to hypomagnesemia.2(11)
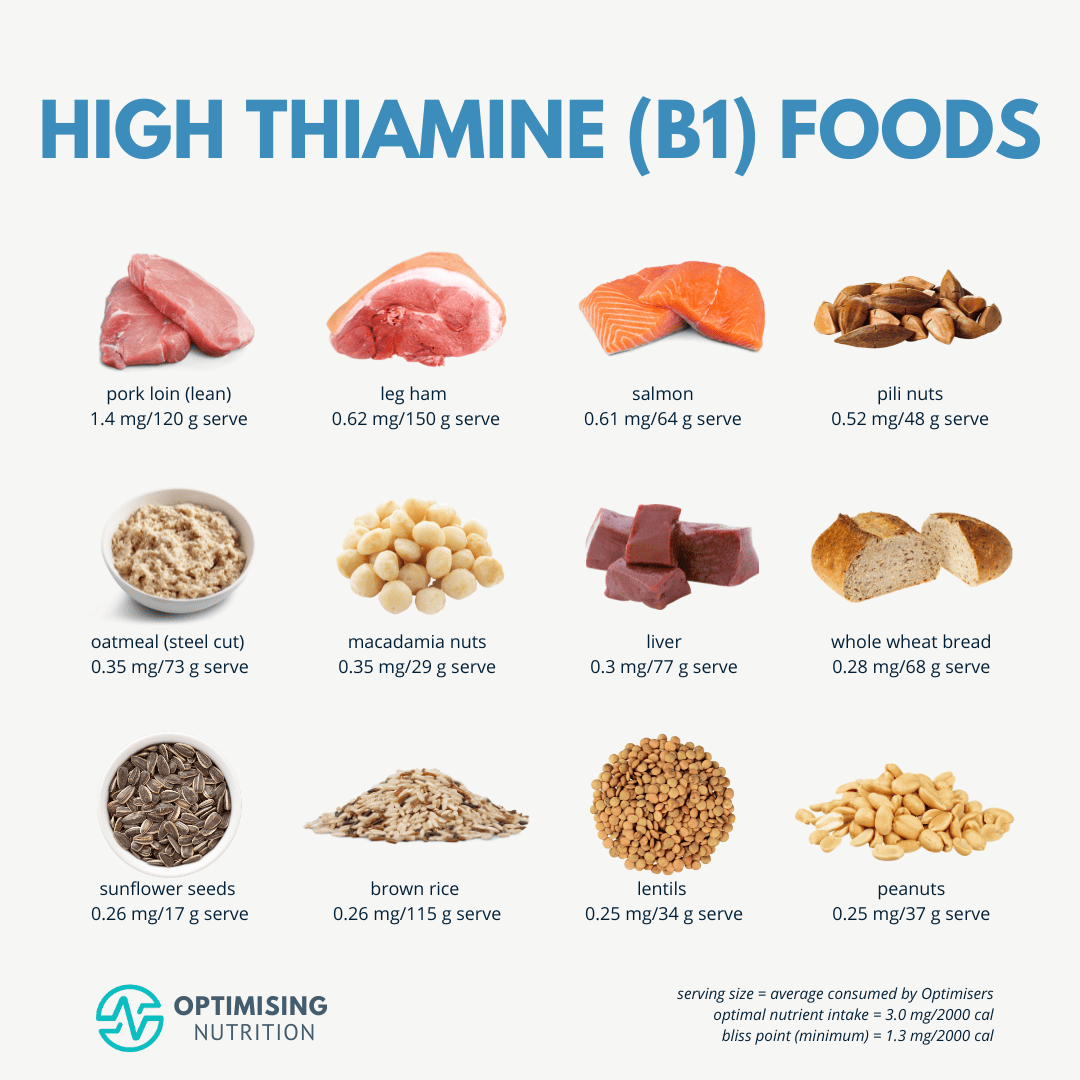
Thiamin (B1) is a third micronutrient that can become deficient among users of AHDs. Mohn et al2(11-12) stated that acute doses of loop diuretics can increase excretion of B1 and is particularly prevalent within elderly (70+ years of age) populations. Such deficiency is compounded in said age group since those in advanced age tend to eat less due to changes in appetite; such a population has been shown to be 2.3 to 4.2 times more likely to have dietary intakes below recommended dietary allowances.2(12) Finally, said drug-related deficiencies are prominent amongst users of an AHD known as furosemide.2(12)

Zinc is another micronutrient known to be lower from increased excretion through the kidneys, and is mostly a result of thiazide diuretics.2(12) Urinary zinc depletion through thiazide diuretics can lead to tissue depletion and might be an additive risk factor of deficiency in addition to inadequate intake, diabetes, hepatic cirrhosis, renal disease, and gastrointestinal disorders.2(12) Angiotensin-converting enzyme (ACE) inhibitors also induce zinc losses by chelation/excretion processes. 2(14) Considering that zinc deficiencies can cause several maladies such as increased risk of infections, infertility, impaired brain development, dermatitis, alopecia, low testosterone, and impaired smell/taste, it is imperative that levels are closely monitored during AHD use.4,5

Potassium is the fifth micronutrient that can become deficient from loop diuretics (increasing excretion through the kidneys) and thiazides, which can manifest as hypokalemia.2(12) Conversely, ACE inhibitors and calcium channel blockers (CCBs) can retain potassium potentially inducing a state of hyperkalemia.2(14,15) In the case of ACE inhibitors, the mechanism of action is achieved through an inhibitory effect of aldosterone (expels potassium in the urine) secretion.2(14) Hyperkalemia may also be increased amongst individuals who are also older, have renal disease, diabetes, or who eat potassium-rich foods.2(14)

In conclusion, AH is considered the predominant risk factor for CVD, and is defined as a resting systolic blood pressure of ≥140 mmHg and/or diastolic blood pressure of ≥90 mmHg in adults. Furthermore, the number of individuals with hypertension has increased in a precipitous fashion since 1980, worldwide. Considering that AH is associated with an increased risk of myocardial infarction, congestive heart failure, kidney disease, and stroke, it is imperative to implement strategies which support optimal blood pressure ranges. Although medications can help control and optimize blood pressure, it is critical that health care providers remain vigilant regarding micronutrient deficiency trends associated with AHDs. Such steps can help maintain heart health while mitigating collateral drug-associated nutrient deficiencies by implementing specific food and supplement interventions for the same.
References
1. Lopes S, Mesquita-Bastos J, Alves A, et al. Exercise as a tool for hypertension and resistant hypertension management: Current insights. Integr Blood Press Control. 2018;11:65-71. doi: 10.2147/IBPC.S136028.
2. Mohn ES, Kern HJ, Saltzman E, et al. Evidence of drug-nutrient interactions with chronic use of commonly prescribed medications: An update. Pharmaceutics. 2018;10(1):1-45. doi: 10.3390/pharmaceutics10010036.
3. Crook MA. Zinc deficiency.Nutrition. 2011;27(10):1085-1086. doi:10.1016/j.nut.2011.06.001.
4. Liu YL, Zhang MN, Tong GY, et al. The effectiveness of zinc supplementation in men with isolated hypogonadotropic hypogonadism.Asian J Androl. 2017;19(3):280-285. doi: 10.4103/1008-682X.189621.
5. Gropper SS, Smith JL, Carr, TP. Advanced Nutrition and Human Metabolism.7thed. Boston, MA: Cengage Learning; 2018.
6. Vidmar M, Grzelj J, Mlinaric-Rascan I, et al. Medicines associated with folate-homocysteine-methionine pathway disruption.Arch of Toxicol. 2018;93(2):227-251. doi:10.1007/s00204-018-2364-z.
-Michael McIsaac
