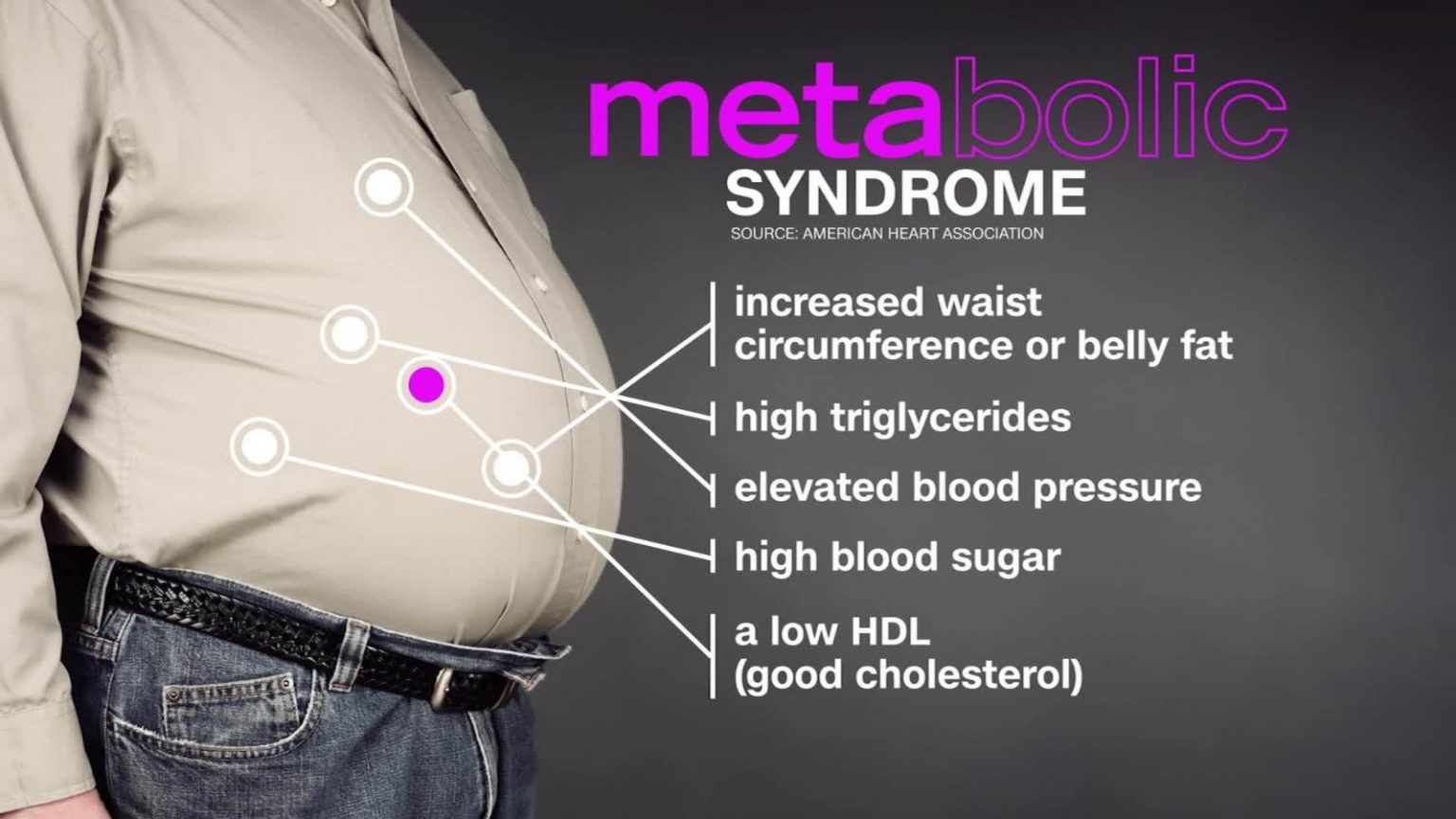
Metabolic syndrome (MS) is a term that identifies an aggregate of physiological abnormalities which increases the risk of liver disease, type 2 diabetes (T2D), and cardiovascular disease (Agyemang-Yeboah, Eghan, Annani-Akollor, Togbe, Donkor, & Afranie, 2019). Physiological abnormalities that constitute MS include dysregulated glucose metabolism, high blood pressure, abdominal obesity, and abnormal lipid profiles (Agyemang-Yeboah et al., 2019). Allopathic interventions have been traditionally used to manage symptoms of MS to include statins, exogenous insulin, blood pressure medication, and bariatric surgery (Gemill, Morgan, & Borys, 2011; Kamran, Ahari, Biria, Malepour, & Heydari, 2014; Mahajan, Gupta, Gupta, & Gupta, 2010). However, the origins of MS can now be traced to lifestyle choices, which includes excessive food consumption, low physical activity, and insufficient sleep quality/duration (Bankoski et al., 2011; Saleh & Janssen, 2014; Song, Lee, Song, Paik, & Song, 2014). Despite the origins and management of MS, early detection is a key logical first step prior to considering interventions. As such, the following will explore biomarkers that can help provide insights into the manifestation of MS, with particular emphasis on dysregulated glucose metabolism.

As mentioned in the first section, MS can be defined as abdominal obesity (waist circumference 102 cm for males, 88cm for females), dysregulated glucose metabolism (≥6.1 mmol/L), high blood pressure (BP ≥130/85 mm Hg) abnormal lipid profiles to include elevated fasting triglycerides (≥1.7mmol/L), and decreased high-density lipoprotein cholesterol (HDL-C, <1 mmol/l for males, <1.3 mmol/L for females) (Lopez-Huertas, 2012). One of the risk factors, dysregulated glucose metabolism/insulin resistance, is a common marker of MS and is thought to play an important role in the pathogenesis of said condition (Jung, Park, Choi, Hong, Choi, & Ryoo, 2017). Moreover, hyperinsulinemia is an early clinical manifestation and biomarker of insulin resistance (Jung et al., 2017). A simple test to track hyperinsulinemia is the insulin assay, which compares glucose load to insulin response in fasted stated and glucose-loaded states (Pagana & Pagana, 2014). Please see below chart for reference. Having briefly considered hyperinsulinemia and its relationship to MS, the following will consider the effects of hyperinsulinemia upon fatty acid profiles.
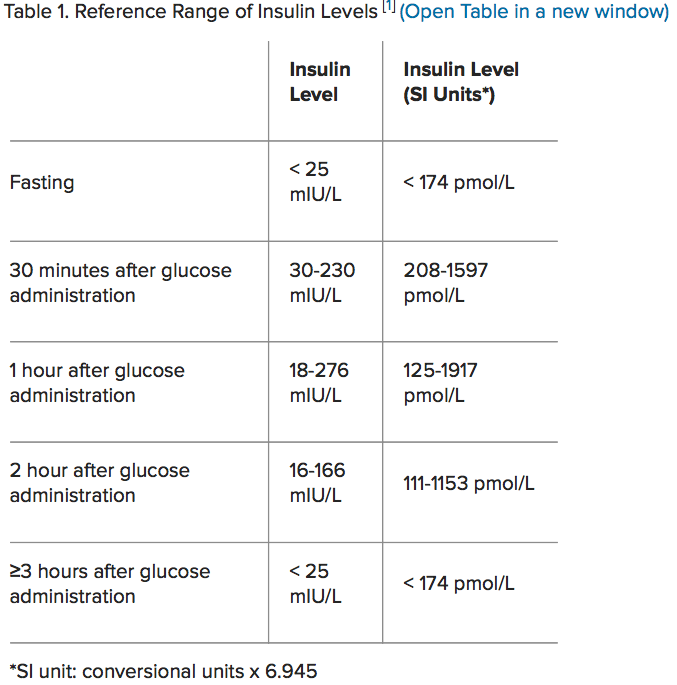
(Medscape, 2014)
Increased serum triglycerides and cholesterol are other biomarkers of interest, which are strongly associated with hyperinsulinemia (Lord & Bralley, 2012). The researchers also explained insulin resistance (cells that only respond to higher than normal levels of insulin) redirects glucose carbon atoms into lipid synthesis, which increases blood levels of fatty acids (Lord & Bralley, 2012). Furthermore, hyperinsulinemia tends to synthesize higher levels of fatty acids such as steric, palmitic, and arachidic acids by down-regulating delta-9 desaturase enzymes. Such enzymes convert saturated fatty acids to monounsaturated fatty acids (Wolters et al., 2015). Higher levels of saturated acids, like palmitic acid, is problematic as it is associated with high triglyceride levels (Lord & Bralley, 2012). Higher triglyceride levels, by itself, is not a constituent of arterial plaque. However, triglycerides do contain cholesterol which is thought to contribute to plaque development (Berglund et al., 2012). Furthermore, triglycerides are a constituent that helps make very low-density lipoproteins (VLDLs), which develop into low density lipoproteins (LDLs); substances considered as atherogenic (Cox & Garcia-Palmieri, 1990).

Another event that can cause aberrations in lipid levels is acetyl CoA carboxylase (ACC) dysregulation. ACC is an enzyme, which facilitates the endogenous production of fatty acids by using substrates found from the citric acid cycle (CAC) (used to produce ATP). Specifically, citrate is converted to acetyl-CoA which is then converted to malonyl CoA, ultimately leading to the synthesis of fatty acids and cholesterol (Tong, 2013). However, conversion of acetyl-CoA to malonyl CoA requires ACC; the first committed step in fatty acid synthesis (Tong, 2013). If there is a deficiency in this enzyme, the production of endogenous fatty acids could become compromised. Tong (2013) did note, however, that it may be beneficial for some individuals to have lower levels of said enzyme. Specifically, the researcher indicated that ACC has been gaining attention as a possible entrance point for pharmaceutical interventions to help control fatty acid/cholesterol, and triglyceride levels amongst individuals with hyperlipidemia. Rat models indicated that down regulated ACC causes a reduction in malonyl-CoA levels, enhances fatty acid oxidation, inhibit fatty acid biosynthesis, improves insulin sensitivity, and reduces plasma triglyceride levels (Tong, 2013). Such outcomes could prove useful amongst individuals with hyperlipidemia and MS, and awareness of ACC levels in such individuals could prove insightful.
In conclusion, MS is an aggregate of physiological abnormalities which increases the risk of liver disease/T2D/cardiovascular disease, and identifying early biomarkers of MS is an essential first step in developing interventions for said condition. Awareness of fasting and post-prandial insulin levels, fasting and post-prandial glucose levels, triglyceride levels, and the influences of key enzymes like ACC can help determine the progression and severity of MS. Ultimately, such an approach (in conjunction to tracking other biomarkers) should inform and encourage individuals afflicted with MS to make necessary dietary and lifestyle changes to manage said condition.
References
Agyemang-Yeboah, F., Eghan, B. A. J., Annani-Akollor, M. E., Togbe, E., Donkor, S., & Afranie, B. O. (2019). Evaluation of metabolic syndrome and its associated risk factors in Type 2 diabetes: A descriptive cross-sectional study at the komfo anokye teaching hospital, Kumasi, Ghana. Biomedical Research International. doi: https://doi.org/10.1155/2019/4562904
Bankoski, A., Harris, T. B., McClain, J. J., Brychta, R. J., Caserotti, P., Chen, K. Y., … Koster, A. (2011). Sedentary activity associated with metabolic syndrome independent of physical activity. Diabetes Care, 34(2), 497-503.
Burglund, L., Brunzell, J. D., Goldberg, A. C., Goldberg, I. J., Sacks, F., Murad, M. H., Stalonhoef, F. H. (2012). Evaluation and treatment of hypertriglyceridemia: An endocrine society clinical practice guideline. The Journal of Clinical Endocrinology and Metabolism, 97(9), 2969-2989.
Cox, R. A., & Garcia-Palmieri, M. R. (1990). Clinical methods: The history, physical, and laboratory examinations (3rded.). Boston: Butterworths.
Gemill, J., Morgan, D. L., & Borys, D. (2011). 71 outcomes and clinical effects from the pediatric ingestion of the cholesterol medication fenofibrate. Annals of Emergency Medicine, 58(4), S201.
Jung, J. Y., Park, S. K., Choi, J. M., Hong, H. P., Choi, Y. J., & Ryoo, J. H. (2017). Evaluation of risk for metabolic syndrome according to the fasting insulin concentration in Korean men. Clinical Chimica Acta, 464, 123-127.
Kamran, A., Ahari, S. S., Biria, M., Malepour, A., & Heydari, H. (2014). Determinants of patient’s adherence to hypertension medications: Application of health belief model among rural patients. Annals of Medical & Health Sciences Research, 4(6), 922-927.
Lopez-Huertas, E. (2012). The effect of EPA and DHA in metabolic syndrome patients: A systematic review of randomized controlled trials. British Journal of Nutrition, 107(s2), 1185-1194.
Lord, R. S., & Bralley, J. A. (2012). Laboratory evaluations for integrative and functional medicine (2nded.). Duluth, GA: Genova Diagnostics.
Mahajan, R., Gupta, A., Gupta, R., S., & Gupta, K. (2010). Efficacy, safety, and cost-effectiveness of insulin sensitizers as add-on therapy in metabolic syndrome in patients with secondary sulfonylurea failure: A comparative study. Journal of Pharmacology and Pharmacotherapeutics, 1(2), 82-86.
Medscape (2014). Insulin. Retrieved from https://emedicine.medscape.com/article/2089224-overview
Pagana, K. D., & Pagana, T. J. (2014). Manual of diagnostic and laboratory tests (5thed.). St. Louis, MO: Mosby.
Saleh, D., & Janssen, I. (2014). Interrelationships among sedentary time, sleep, duration, and the metabolic syndrome in adults. BMC Public health, 14(1), 666.
Song, S., Lee, J. E., Song, W. O., Paik, H. Y., & Song, Y. J. (2014). Carbohydrate intake and refined-grain consumption are associated with metabolic syndrome in the Korean adult population. Academy of Nutrition and Dietetics, 114(1), 54-62.
Tong, L. (2013). Structure and function of biotin-dependant carboxylases. Cellular and Molecular Life Sciences, 70(5), 863-891.
Wolters, M., Schlenz, H., Börnhorst, C., Risé, P., Galli, C., Moreno, L. A., … Tornaritis, M. (2015). Desaturase activity is associated with weight status and metabolic risk markers in young children. The Journal of Clinical Endocrinology and Metabolism, 100(10), 3760-37 69.
-Michael McIsaac
