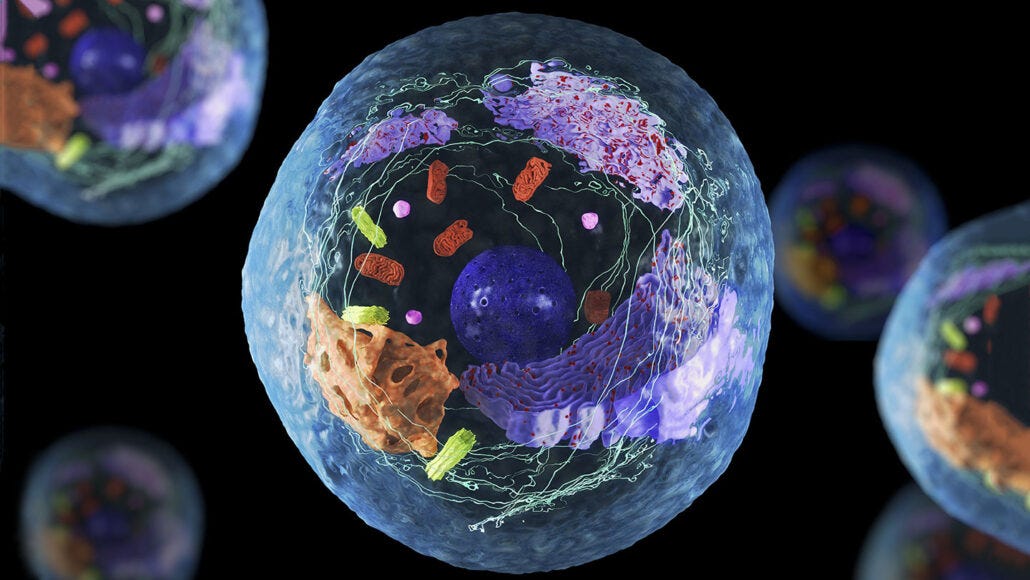
Cells represent the basic functional units of an organism (Reisner, Reisner, & Crowley, 2017). Comprehending the underlying nature of cell function, and dysfunction, provides insights into the homeostasis of the organism as a whole. As a means of appreciating cell behavior, the following sections will provide a brief overview of mechanisms behind stem cell aging and damage.
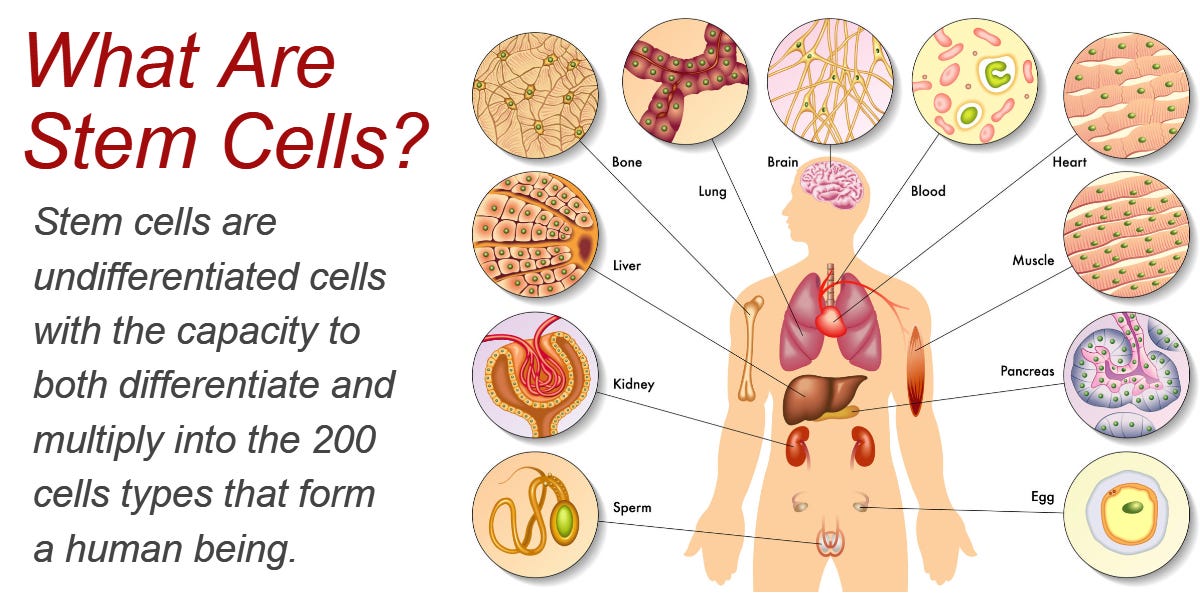
Stem cells, which can self-renew and differentiate into other cell types, helps regenerate damaged tissues throughout the body (Schultz & Sinclair, 2016). Although stem cell phenotypes have different rates of aging, Schultz and Sinclair (2016) noted that there does exist several commonalities: stem cells have telomerase (required for self-renewal), there are unique metabolic requirements, stem cells distribute macromolecules asymmetrically, and they reside in niches that regulate cell behavior.
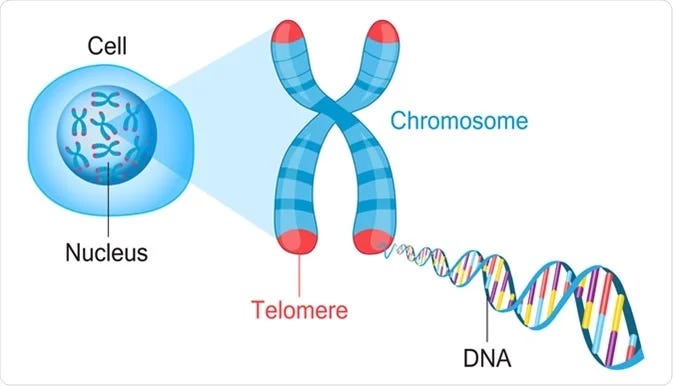
Telomeres are a region of repetitive nucleotide sequences at each end of a chromosome, which protects the end of the chromosome from deterioration (Schultz & Sinclair, 2016). The researchers stated there is a correlation of telomere length and survival of the elderly in old age. Additionally, telomere length predicted survival in elderly twins suggesting that telomeres contribute to health and longevity of humans, while controlling for genetic influences (Schultz & Sinclair, 2016). Thus, protection of telomere length is paramount.
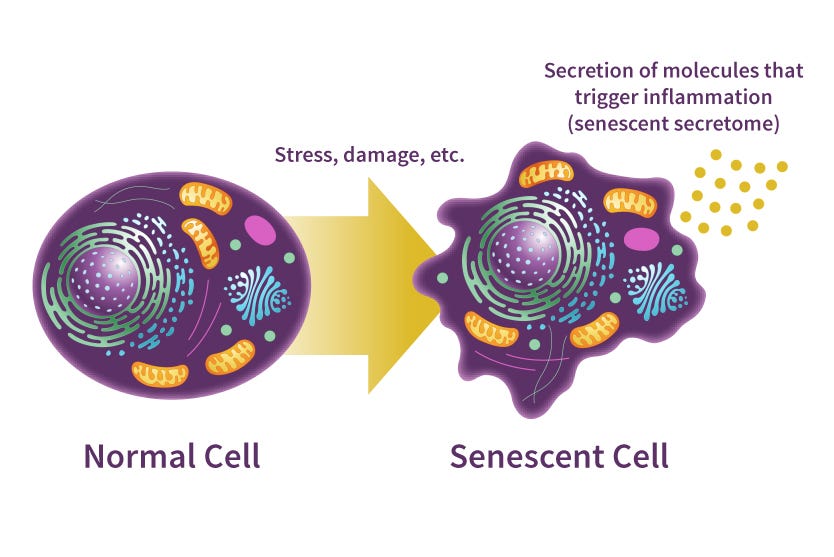
Stem cells can also experience senescence; a gradual loss of function from deterioration (Schultz & Sinclair, 2016). Such loss occurs from telomere shortening and/or uncapped telomeres, and is strongly associated with stem cell aging. Moreover, dysfunctional stem cells may negatively influence other stem cells in close proximity via secretion of tumor-promoting mitogens (chemical substance) and pro-inflammatory cytokines (cell signaling molecules) (Schultz & Sinclair, 2016). Therefore, controlling the onset of senescence of stem cells may help protect nearby stem cells from the same fate.
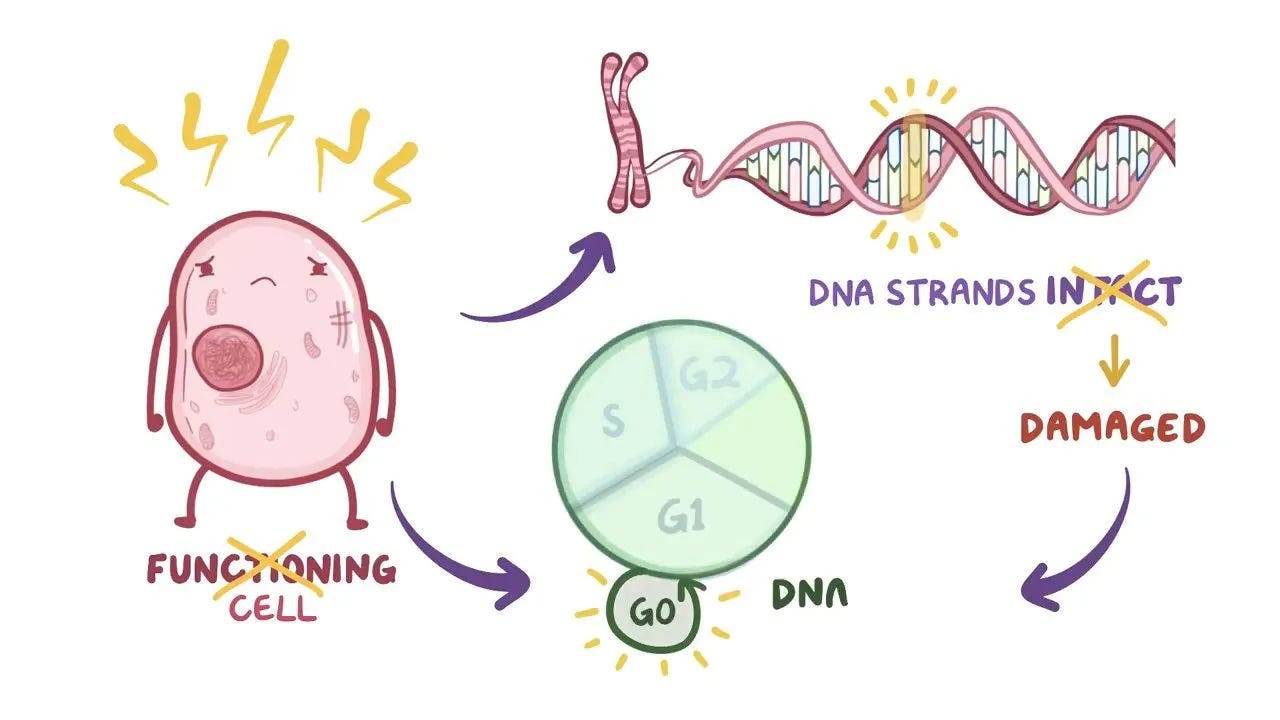
DNA damage and mutations are also connected to stem cell aging. Markers of DNA damage (H2AX phosphorylation and comet tails) have been shown to increase with cell age (Schultz & Sinclair, 2016). Consequences of DNA damage include an increased rick of developing cancerous cells. Additionally, DNA damaged stem cells may also undergo cell death (apoptosis), senescence, or differentiation (Schultz & Sinclair, 2016).
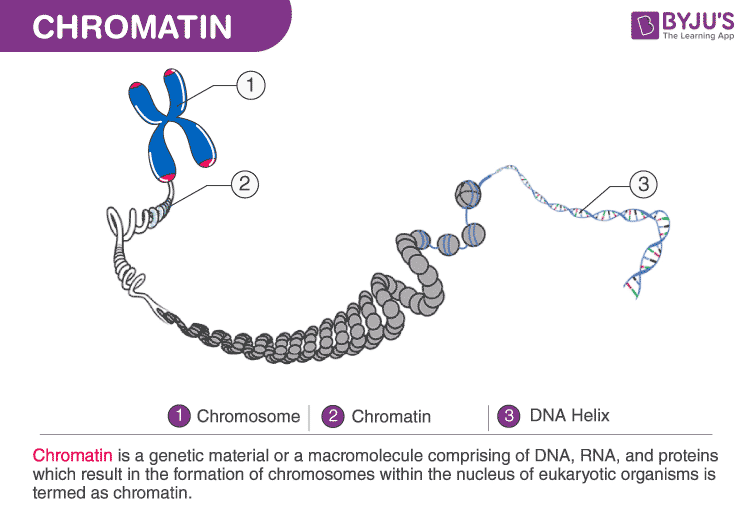
Chromatin is a complex of macromolecules, which compacts and reinforces DNA, controls gene expression, and prevents DNA damage. Interestingly, Shultz and Sinclair (2016) noted that as a cell ages from stress, chromatin also undergoes permanent changes, especially from DNA damage, such as a double strand break (DSB). DSBs are particularly hazardous to the stem cell because it can lead to genome rearrangement. Schultz and Sinclair (2016) noted that chromatin never fully repairs from DSBs, which may have a deleterious effect on newly formed stem cells.
In conclusion, there are many processes within a cell that must be regulated to maintain homeostasis and longevity for the organism. Deviations of normal cell regulation can lead to both minor and major changes in cell function and health. Ultimately and most importantly, appreciating the pathophysiology of the cell serves as a foundation for improving the health, performance, and longevity of the individual as a whole.
References
Reisner, E. G., Reisner, H. M., & Crowley, L. V. (2017). An introduction to human disease: Pathology and pathophysiology correlations (10th ed.). Burlington, MA: Jones & Bartlett Learning.
Schultz, M. B., & Sinclair, D. A. (2016). When stem cells grow old: Phenotypes and mechanisms of stem cell aging. Development, 143(1), 3-14.
-Michael McIsaac
