
Fat is a macronutrient necessary for several key physiological roles in the body. Specifically, fatty acids (carbon bonds ranging from 4-24 with a carboxyl group) which form fats, is a large energy source and provider of cell membrane constituents (Calder, 2015; Lord & Bralley, 2012). Fatty acids (FAs) also have biological roles that include tissue and cell structure/metabolism, function, and responsiveness to hormonal/other signals (Calder, 2015).
FAs also influence and facilitate intracellular signaling, gene expression/transcription, and regulation bioactive lipid mediators (Calder, 2015). Therefore, FA dysregulation has been implicated with several disease states to include obesity, cancer, diabetes, cystic fibrosis, heart disease, arthritis, and multiple sclerosis (Lord & Bralley, 2012). As a means of appreciating FA energy production, the following will consider a subject’s organic acid testing profile of the same, and its implications upon health and homeostasis.
FAs are transported in the blood via lipoproteins or as free FAs bound to albumin. Once in contact with the cell membrane, FAs are released from their carriers and prepare for entry via cell membrane transport proteins (Lord & Bralley, 2012). Once inside, FAs are released from their transporters and enter the transmembrane space of the mitochondria (TMSM). FAs are then attached to coenzyme A (CoA) via acyl-CoA synthetase (AS). If FAs are medium chain (6-12 carbons), they can readily pass through the outer mitochondrial membrane (OMM).
However, if FAs are long chain (over 13 carbons), they require attachment to carnitine via carnitine acyl transferase (CPT1) producing fatty acyl carnitine. Fatty acyl carnitine then moves past the IMM via translocase enzyme (TL) (Lord & Bralley, 2012). In essence, a complex set of processes must take place in order for FAs to gain entry and be used as energy substrates in the CAC. Please see below illustration:
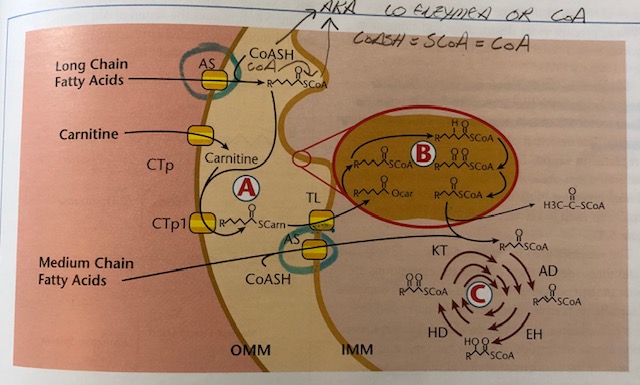
(Lord & Bralley, 2012)
If FAs are being used inefficiently, there are biomarkers in the form of organic acids (OAs), that can help elucidate the degree and location of FA dysregulation. Such biomarkers include adipate, suberate, and ethylmalonate (Lord & Bralley, 2012). Please see illustrations below for reference:
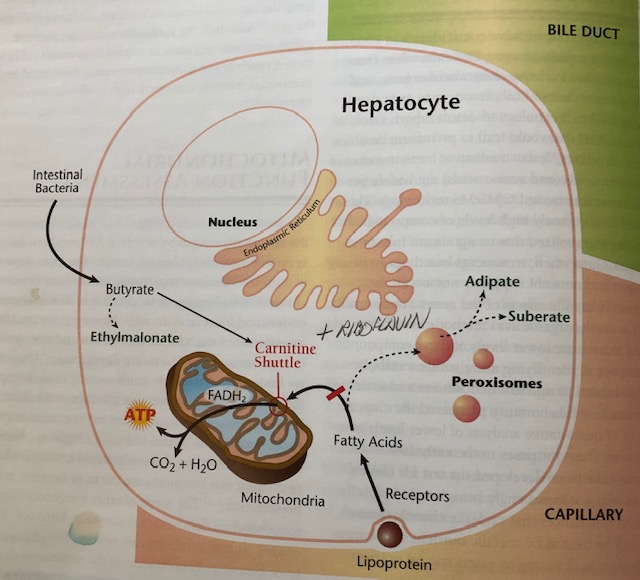
(Lord & Bralley, 2012)
Since carnitine is necessary to transport long and very long chain FAs, if there is low carnitine (or low lysine from which carnosine is produced), entry of FAs into the CAC will become compromised (Lord & Bralley, 2012). A compensatory mechanism in the presence of low carnitine/low mitochondrial oxidation uses peroxisomes to continue producing energy (see previous illustration) (Lord & Bralley, 2012).
However, adipate and suberate (6 and 8 carbon long dicarboxylic acids, respectively) are produced as a by-product of this energy-producing pathway. Thus, the presence of said dicarboxylic acids indicate low carnitine availability (Lord & Bralley, 2012). Another cause of high adipate and suberate in the urine is riboflavin (B2).
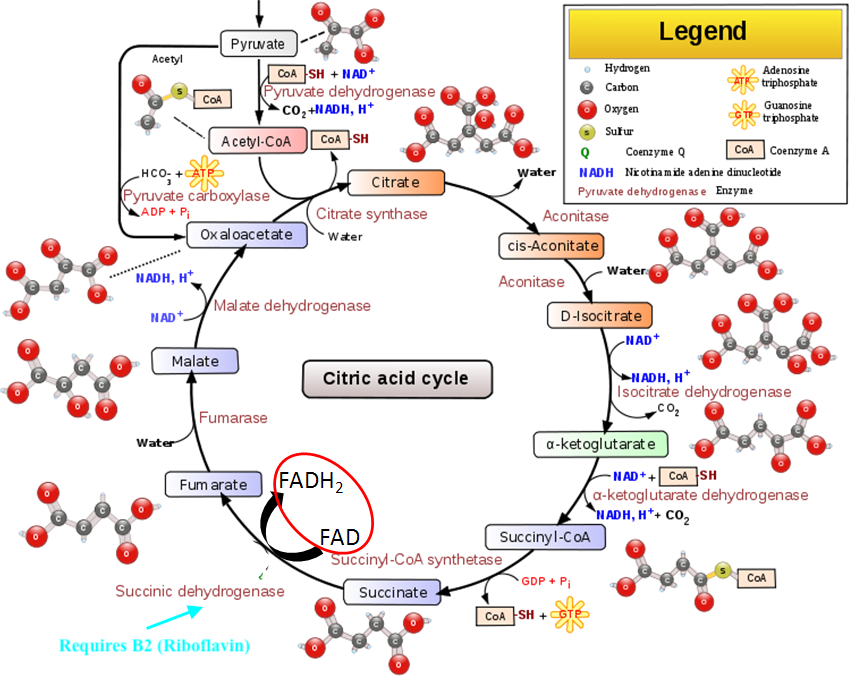
Within the CAC, there are 9 intermediates necessary to metabolize acetyl-CoA, in addition to the electron transport chain (ETC), which ultimately produce ATP. An enzyme, known as succinate dehydrogenase (SD), is used in both in the CAC and ETC (Lord & Bralley, 2012). However, said enzyme requires the presence of B2 to produce flavin adenine dinucleotide (FAD).
Thus, if B2 is low, the CAC/ETC ATP production can slow, again necessitating the alternative ATP production route via peroxisomes FA oxidation. Such a process produces adipate and suberate as by-products. Individuals with low B2/carnitine may present with levels outlined below:
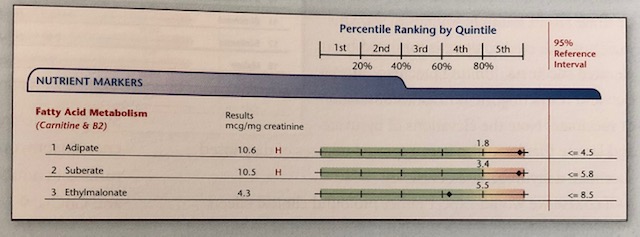
(Lord & Bralley, 2012)
In addition to elevated adipate and suberate, ethylmalonate (EM) may also become elevated, though it is not an indicator of peroxisome FA oxidation. Rather, EM is an organic acid which indicates/is hypothesized to derive from butyrate, a short chain fatty acid (less than 6 carbons), produced by intestinal bacteria (Lord & Bralley, 2012; Schonfeld & Wojtczak, 2016).
Similar to long chain fatty acids, carnitine deficiency can hinder its entry into the matrix of the mitochondria, denying its use in the CAC. Low B2 can also hinder butyrate metabolism within the CAC and ETC (Lord & Bralley, 2012). Instead, butyrate is converted to EM indicating a deficiency in carnitine and B2 availability. EM may also become elevated if there is a genetic polymorphism in the enzyme responsible for oxidizing the same; short-chain acyl-CoA dehydrogenase (SCAD) (Lord & Bralley, 2012).

The last illustration/example also outlined low lysine and low branched chain amino acids (BCAAs). Low lysine, as mentioned earlier, is a precursor for carnitine synthesis. Thus, increasing lysine via supplementation (i.e., two doses/day), and food sources, may help produce carnitine (Lord & Bralley).
Furthermore, low B2 is a secondary cause of high adipate/suberate/ethylmalonate, and supplementation of the same may also be indicated to help ensure there is adequate FAD production to maintain CAC and ETC ATP production (Lord & Bralley, 2012). Low BCAAs (leucine, valine, isoleucine) may likely be from low dietary protein intake, and such low levels are thought to contribute to aberrations in neurotransmitter synthesis and brain function (Schonfeld & Wojtczak, 2016).
In conclusion, FAs support several key physiological roles to include ATP production, components for cell membrane development, enhancing tissue and cell structure/metabolism, function, and responsiveness to hormonal/other signals (Calder, 2015). As such, aberrations in FA metabolism can interrupt homeostasis; a process which can be tracked and controlled through adipate/suberate/ethylmalonate organic acid testing in addition to monitoring lysine and B2 levels.
References
Calder, P. C. (2015). Functional roles of fatty acids and their effects on human health. Journal of Parenteral and Enteral Nutrition, 39(1), 18S-32S.
Lord, R. S., & Bralley, J. A. (2012). Laboratory evaluations for integrative and functional medicine (2nded.). Duluth, GA: Genova Diagnostics.
Schonfeld, P., & Wojtczak, L. (2016). Short- and medium-chain fatty acids in energy metabolism: The cellular perspective. Journal of Lipid Research, 57(6), 943-954.
-Michael McIsaac
