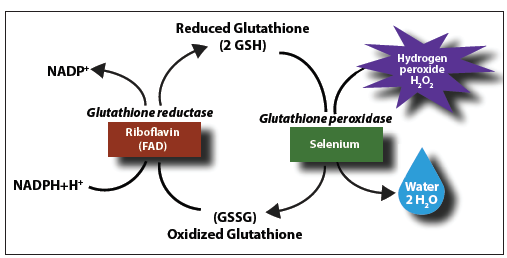
It is thought that reactive oxygen species (ROS) are involved in many disease states to include arthritis, cancer, cardiovascular disease, diabetes, and Alzheimer’s disease (Duthie, 2003; Stockler-Pintos, Mafra, Farage, Boaventura, & Cozzolino, 2010). As such, maintaining optimal levels of anti-oxidants are critical in managing the destructive nature of ROS. Glutathione peroxidase (GSH-PX) is an example of an enzymatic anti-oxidant, which serves to protect cellular membranes (i.e., the lipid portion) and other structures from ROS damage. However, it shares an intimate and necessary relationship with selenium (Se) to carry out such duties. The following will explore said relationship as well as optimal sources of Se, and its affects upon Se deficiency.

Stockler-Pintos et al. (2010) posited that ROS could be related to the manifestation CVD. Furthermore, said researchers proposed that Se supplementation could assist in the regeneration of GSH-PX (see diagram below) by increasing concentrations of said trace mineral. Long-term Se deficiency is related to a decrease in GSH-PX activity, which is thought to provide opportunity for ROS-related cellular damage (Stockler-Pintos et al., 2010). As such, Stockler-Pintos et al. (2010) proposed implementation of a whole food (Brazil nuts) dense in Se instead of supplementation, since there is a lower risk of experiencing toxicity. The supplemental form of Se is sodium selenite (SS). However, research has indicated that the Brazil nut has a bioavailability similar to that of SS (Stockler-Pintos et al., 2010). As such, the Brazil nut provides a natural source of Se, and one that is cheaper than the synthesized form.

The researchers considered 81 patients (55 males and 26 females) who required hemodialysis; a condition known to increase ROS aggregation, and thought to be the result of chronic inflammatory states, advanced age, and high frequency of diabetes (Stockler-Pintos et al., 2010). Baseline measures of plasma Se levels, erythrocyte levels, and GSH-PX activity were measured before the intervention. Upon completion of said baseline measures, each patient received one Brazil nut/day for 3 months. Such a feeding period was chosen as Stockler-Pintos et al. (2010) indicated that European Best Practice Guidelines suggested said period to allow time to increase plasma levels.
Brazil nut composition was maintained for consistency to include 0.45 g of carbohydrates, 0.75g of proteins, and 3.53 g of lipids (Stockler-Pintos et al., 2010). 3 months later, measures were taken again (plasma Se levels, erythrocyte levels, and GSH-PX activity) and compared to baseline. The national Nutrition Survey indicated that recommended Se levels for males included 56 µg and females as 39µg(Stockler-Pintos et al., 2010). Baseline measures of plasma Se was 18.8 +/- 17.4 mg/L, while levels were 104.0 +/- 65.0 mg/L after the intervention. Furthermore, erythrocyte levels were 72.4 +/- 37.9 mg/L pre-intervention, and increased to 244.1 +/- 119.5 mg/Lpost-intervention(Stockler-Pintos et al., 2010). Finally, GSH-PX activity before Se consumption (in 11% of the patients) was 27.5–73.6 U/g of hemoglobin. Post intervention levels increased to 55.9 +/- 23.6 U/g of hemoglobin (Stockler-Pintos et al., 2010).
In conclusion, evidence has supported the positive impact of specific foods on Se levels and anti-oxidant activity. Most relevantly, the research of Stockler-Pintos et al. (2010) emphasized the impact of Brazil nut consumption in rectifying Se deficiency; a modicum of said nut (i.e., one nut/day) for 3 months demonstrated a positive and measurable change in biomarkers central to maintaining optimal Se levels. Finally, the study is a reminder of the influence of food as a first line intervention (i.e., food-first approach) in addressing health-related matters in a safe and cost-effective manner.
References
Duthie, G. (2003). Antioxidants. Medicine, 31(4), 25-26.
Stockler-Pintos, M. B., Mafra D., Farage, N. E., Boaventura, G. T., & Cozzolino, S. M. F. (2010). Effect of Brazil nut supplementation on the blood levels of selenium and glutathione peroxidase in hemodialysis patients. Nutrition, 26(11), 1065-1069.
-Michael McIsaac
