
Ethical guidelines in human research finds its beginnings shortly after the Second World War. A cornerstone of ethical research is informed consent, and a fundamental principle of ethical standards is to treat human participants with respect while protecting individual rights. However, these guidelines did not exist prior to 1946. In the following sections, I would like to examine human research methods prior to 1946, the genesis of informed consent, and a review of its contemporary elements.

Pick, Berry, Gilbert and McCaul (2013) noted that prior to 1946, abuse of participants in human-centered research was common, and that little existed in the way of written ethical codes. An example that continues to echo and resonate from the past is the medical research performed upon prisoners in Nazi concentration camps. In response to the cruel and unusual treatment of human subjects during the Second World War, the Nuremburg Code was created, outlining steps and guidelines to protect the welfare of human subjects involved in research (Pick et al., 2013).
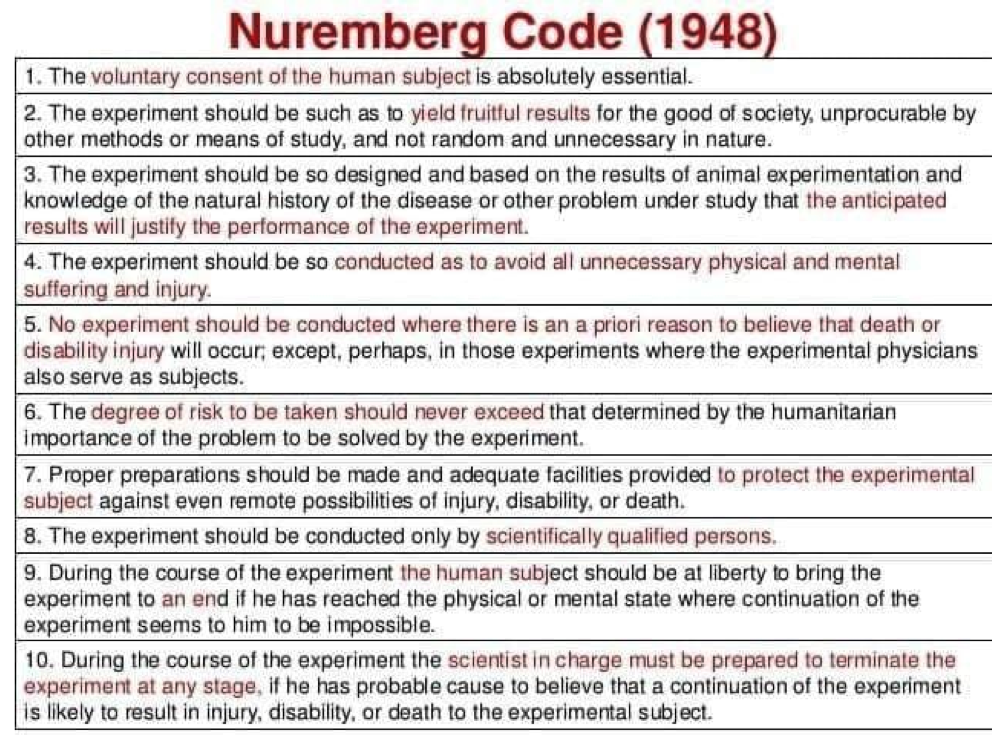
The most relevant concept of the Nuremberg Code is that of informed consent. Pick et al. (2013) stated that informed consent was essential, and that it is only legally valid and professionally acceptable when based on specific criteria: a participant has the capability to decide whether to take part in the research, the participant has been properly informed and educated, and has not been pressured or coerced to participate. This was considered to be the most significant document in the history of medical research ethics (Pick et al., 2013). The following sections will explore the elements of informed consent in more detail.

Informed consent should be preceded by adequate disclosure of information. Information about the research must be clear and accurate. The information must also outline how the research could potentially benefit the participant, as well as a clear outline of any potential hazards or risks (Pick et al., 2013). Disclosure of information also contains within it, what will happen to the research participants when they commence the experiment: how long they will be in the experiment, what they are expected to do, where and how their personal information and data will be managed and stored. Finally, if the participants choose to withdraw from the experiment at any point in time, they will still receive any beneficial treatments that have been supported by the research (Pick et al., 2013).

The second element of informed consent is the patient information sheet. This document, which has been approved by the research ethics committee, outlines the main points of the study. The leaflet should also be easy to read and understand with simple terminology. It may also include illustrations as well as contact information, so that the participant may be free to acquire additional information (Pick et al., 2013). Pick et al. (2013) also noted that the participants should have adequate time to think about, and decide if they wish to participate.

The consent form constitutes the third element of informed consent. Pick et al. (2013) stated that consent could be given verbally or non-verbally. Additionally, it is not a legal requirement to complete a consent form before engaging in research, though it is considered best practice (Pick et al., 2013). Of particular interest is having a participant sign an informed consent form does not constitute legal validity (Pick et al., 2013). Researchers should follow signed informed consent documents with written summaries outlining all information shared with the participant, to help substantiate that the researchers moved above and beyond minimal requirements of informed consent (Pick et al., 2013).

The final element of informed consent is the capacity to consent. The capacity to consent is particularly relevant when recruiting children, individuals with learning disabilities, offenders, ethnic minorities and individuals with a poor comprehension of English (Pick et al., 2013). Each of the aforementioned examples contain vulnerabilities because there may be barriers that would keep the person from true comprehension of the research. For example, children who are under the age of sixteen are only permitted to engage in informed consent if they can clearly demonstrate insight and comprehension of the elements of the research (Pick et al., 2013). Pick et al. (2013) outlined the importance of including the parents or legally appointed guardians throughout the entire process, to help ensure the researcher is upholding the welfare and interests of the participant.
Research allows a platform to discover and uncover knowledge that can enrich and help individuals and societies in a myriad of ways. However, the research community must strike a balance between the urgency for new information and potential risks and benefits bestowed upon research participants. The cornerstone of ethical research is informed consent, and its existence must be guarded, scrutinized and continually refined to maintain the foundations upon which ethical research is based.
References
Pick, A., Berry, S., Gilbert, K., & McCaul, J. (2013). Informed consent in clinical research. Nursing Standard, 27(49), 44-47.
-Michael McIsaac
