Blood biomarkers such as high triglycerides, high low-density lipoprotein (LDL)/high-density lipoprotein (HDL) ratios, high ferritin, high homocysteine, and low alpha-tocopherol levels have been linked to hypertension and cardiovascular disease (CVD) states. Furthermore, such blood markers are also quite common within blood chemistry panels. As a means of appreciating said markers and their relationship to high blood pressure/CVD, the following will explore the same in greater detail, as well as nutritional and supplemental interventions to support normalization of such pathophysiological events.
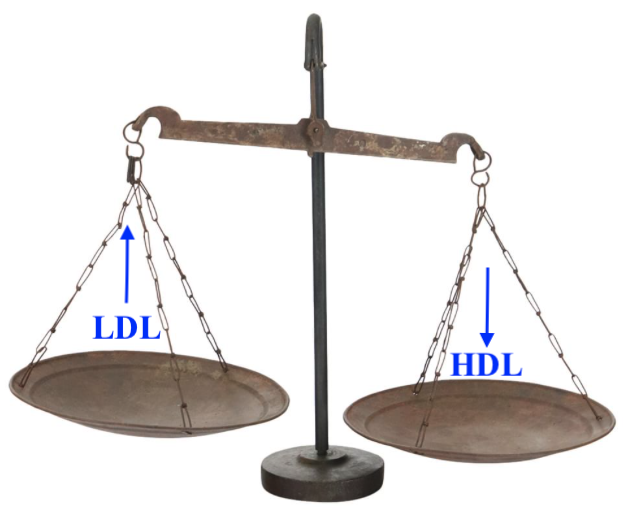
High LDL/HDL ratios are associated with chronic inflammation; a condition associated with many disease states to include diabetes, cancer, arthritis, osteoporosis, mental health, macular degeneration, coronary heart disease, and essential hypertension.1 Unlike high ferritin (an aforementioned indicator of acute phase inflammation), research indicates that high LDL/HDl ratios not only serve as a marker chronic inflammation; they also participate as an underlying impetus behind such a condition.1(677)
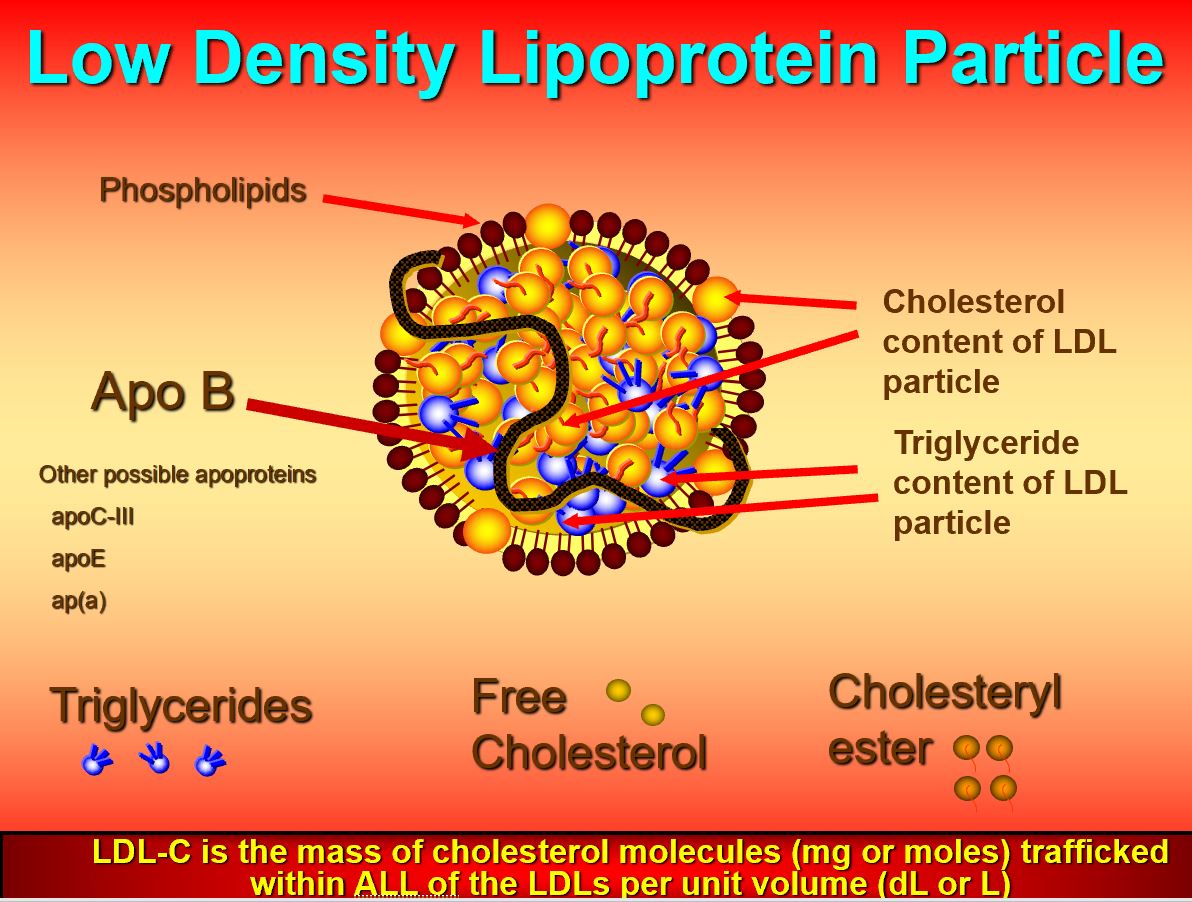
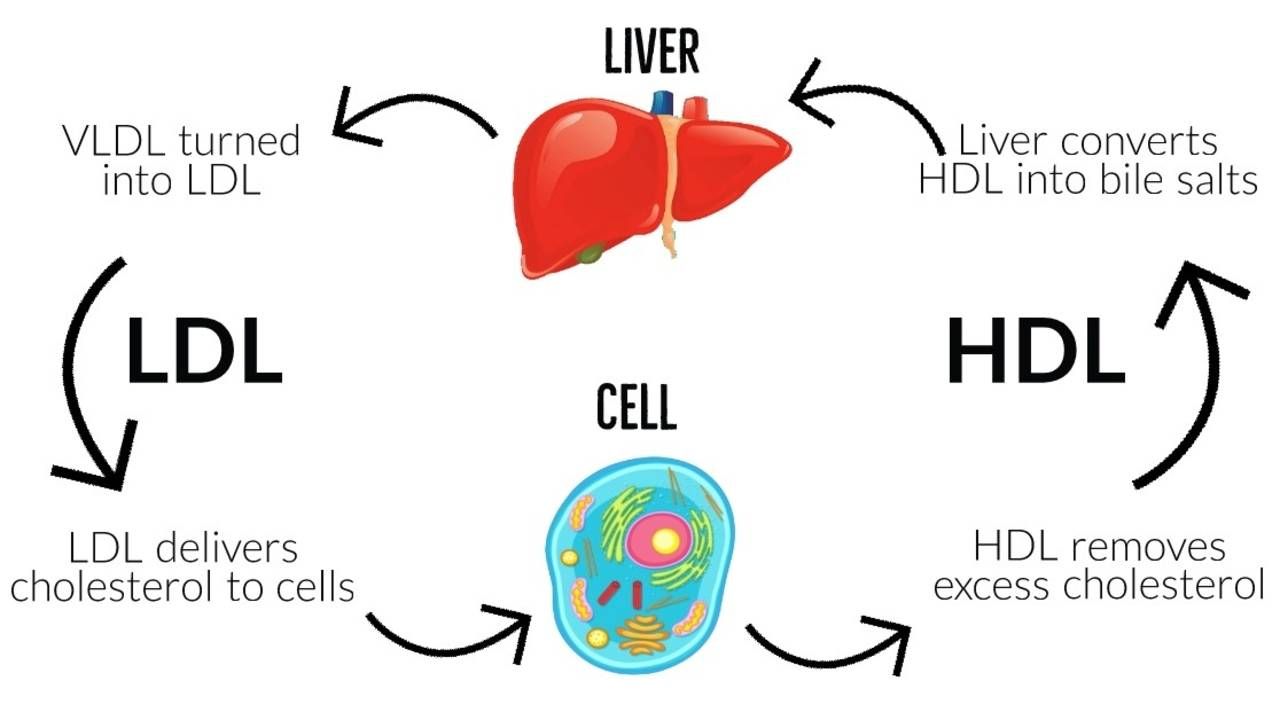
LDLs, as explained in previous posts, act as transporters of cholesterol, and triglycerides; LDLs carry said lipids in a form that allows for successful transport and deposition throughout the blood, cells, tissues, and organs of the body.2 Furthermore, cholesterol is a lipid that is critical for the structure of cellular membranes, is a precursor for steroid hormones and vitamin D, and is used by the liver to produce bile acids. However, mismanagement/misallocation of cholesterol via excessive LDL production can induce unfavourable outcomes to include atherosclerosis and hypertension.
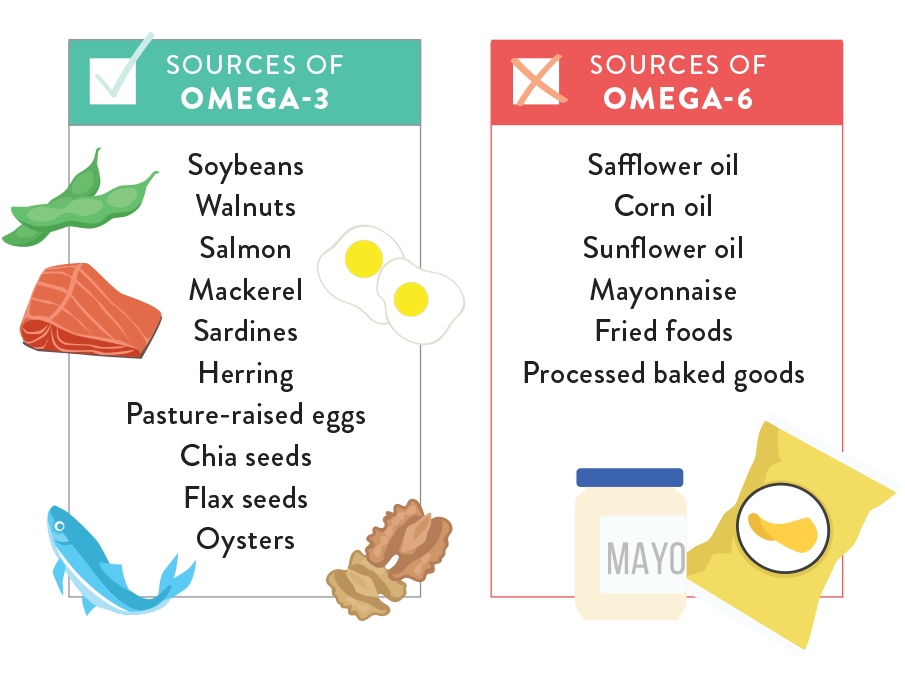
One driver behind excessive LDL production, and increased LDL/HDL ratios, is excessive consumption of omega-6 fats, trans fatty acids, and production of high triglycerides from processed foods and oils.1(674-675) Diets rich in omega-6 fatty acids, such as linoleic acid, are incorporated into LDLs which increases their dominance over HDLs. High triglycerides (caused by excessive consumption of refined carbohydrates) also tend to convert into LDLs.3 Finally, the presence of linoleic acids within LDLs also increases the susceptibility of said cholesterol transporter to oxidative stress; a process which ultimately leads to the deposition of plaques along arterial walls.1(678)
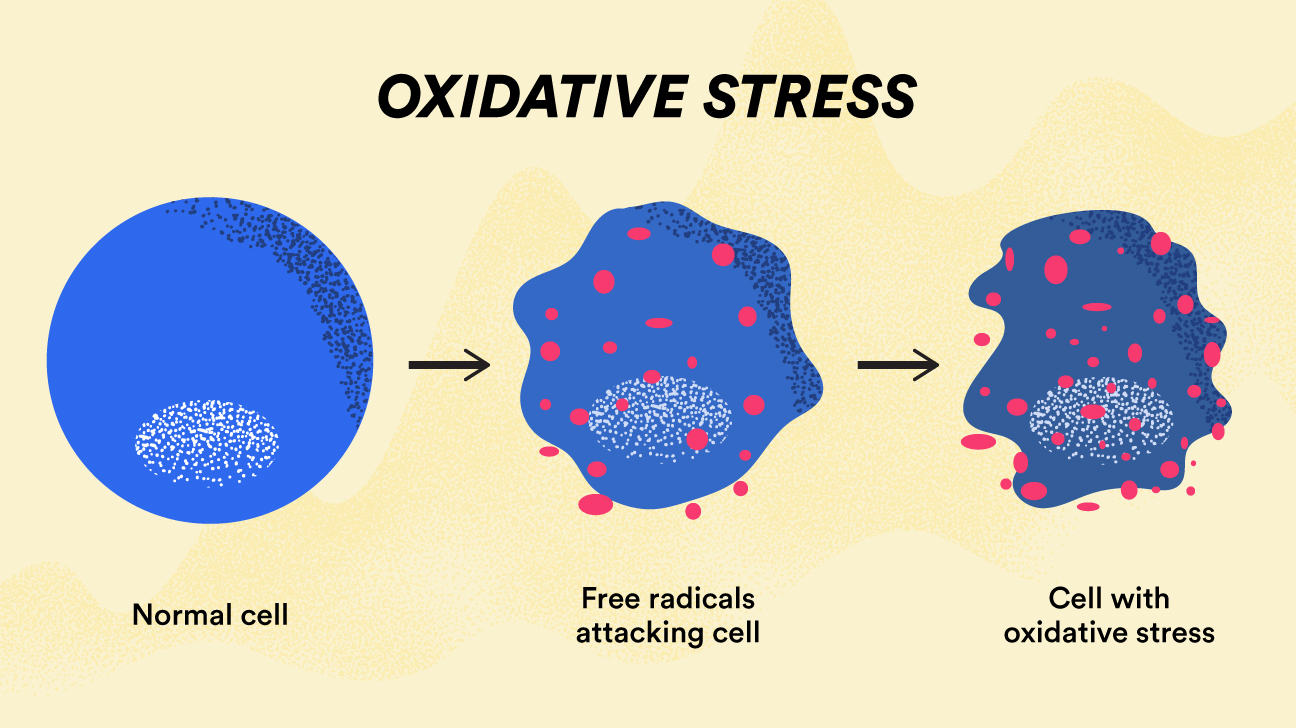
Free radicals, a consequence and product of cellular metabolism, are highly reactive molecules that if left unchecked, interact with and damage nearby cells/lipid membranes/enzymes/DNA.3 Free radicals impose their ability to disrupt cellular function through unpaired electrons in their outermost orbital shells.3(515) Upon contact with a cellular target, free radicals extract electrons from said cellular components, inducing a state of oxidation. Oxidized cells/organelles, by consequence, become unstable and dysfunctional. Such markers of oxidative stress are indicated by elevated homocysteine levels in the blood.
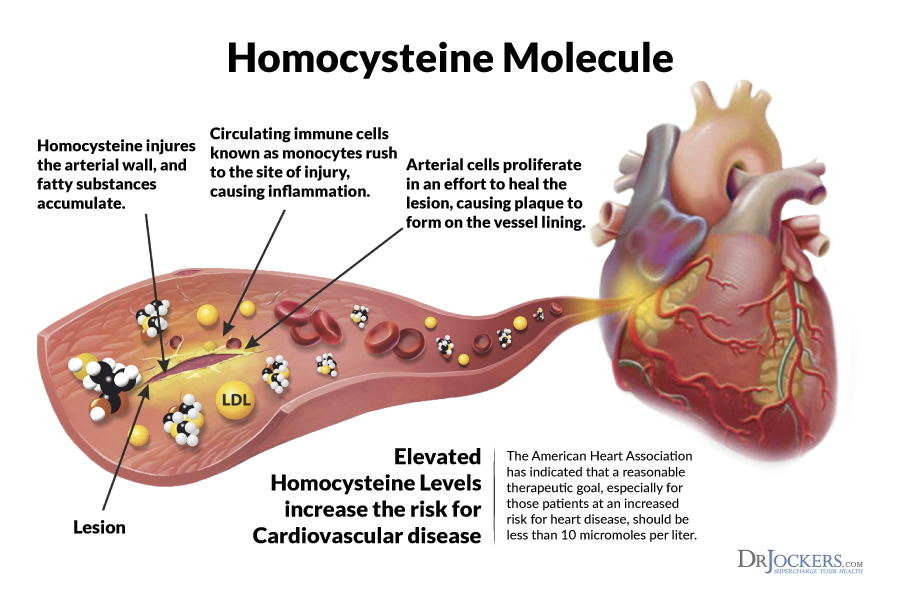
Homocysteine, like LDLs, are not only a marker of an aberration in health; they can also be the progenitor of the same.5 Homocysteine is an amino acid that, at high concentrations, can increase oxidative stress, damage blood vessel linings, and potentially induce inflammation.5(579) Homocysteine participates in the production of methionine (helps activate other molecules) and cysteine (a pre-cursor to glutathione; a strong anti-oxidant). However, if other micronutrients responsible in converting homocysteine to methionine/glutathione are low such as B12, B6, and folate, homocysteine levels can rise.
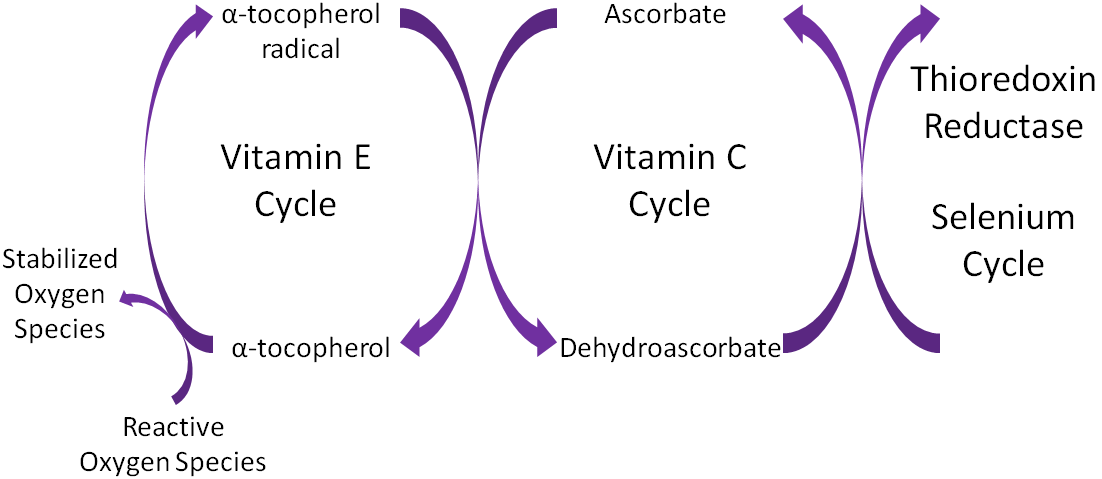
Another marker of oxidative stress is low alpha tocopherol, as indicated in the clients’ blood chemistry panel. As in the cases of high homocysteine and high LDLs, low alpha tocopherol not only marks poor health; said micronutrient also participates in pathophysiological processes. Alpha tocopherol (vitamin E) is an antioxidant, which donates electrons to free radicals thereby neutralizing downstream effects of oxidation.6 Specifically, vitamin E contains a hydroxyl group which donates hydrogen ions to the free radical and reduces/inhibits the oxidation of fragile cell membrane structures.6(404) Once enough hydroxyl groups are presented to a free radical, cell membrane destruction decreases; a process known as termination.6(404) Thus, if vitamin E levels are low, opportunities for oxidative processes are more likely.

Solutions to decrease LDL concentrations would include lowering omega-6 fatty acids in the diet thereby lowering the LDL/HDL ratios. Such a change decreases vascular damage and atherschlerosis.1(679) Reduction of omega-6 fatty acids can be attained by avoiding processed foods, which are known to contain high concentrations of said fatty acids, and also responsible for the production of triglycerides.7 Furthermore, increasing foods rich in omega-3 fatty acids also facilitates reductions in inflammation and improvement LDL/HDL ratios, since omega-3 fatty acids down regulate inflammatory cytokines.1(680) Sources of omega-3 fatty acids include fatty fish and fish oil supplementation, if access to food sources are limited.8

Oxidative stress can be reduced via increasing vitamin E food via food sources such nuts, seeds, olive oil, and leafy green vegetables.8(14) If blood panel vitamin E markers continue to remain low, supplemental versions of the same can be implemented to reach optimal blood concentrations. Vitamin B12, as mentioned in previous sections, can also assist in reducing homocysteine levels, and said micronutrient can be found in most animal products with particularly high concentrations in beef, lamb, and fish.9 If B12 continues to remain low, supplementation might be indicated. Folate (B9) can also help reduce homocysteine levels, and can be found in green leafy vegetables, meat, and dairy. Similar to B12, if blood chemistry panels continue to indicate low folate, supplementation can be introduced to improve levels.10 Finally, pyridoxine B6 has also been shown to reduce homocysteine levels and can be found in sunflower seeds and beef liver. Furthermore, supplemental B6 can be implemented if deemed necessary.8(181)
In conclusion, blood biomarkers such as high triglycerides, high low-density lipoprotein (LDL)/high-density lipoprotein (HDL) ratios, high ferritin, high homocysteine, and low alpha-tocopherol levels have been linked to hypertension and cardiovascular disease (CVD) states. However, implementation of foods rich in omega-3 fatty acids, vitamin E, B9, B6, and B12 can help circumvent such deficiencies while improving said risk factors for hypertension and CVD. If using a rood-first approach does not improve said markers, supplementation of such nutrients can help facilitate a return to optimal levels.
References
1. Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med. 2008;233(6):674-688. doi:10.3181/0711-MR-311.
2. Lee RD, Nieman DC. Nutritional Assessment. 6th ed. New York, NY: McGraw-Hill; 2013.
3. Lord RS, Bralley, JA. Laboratory Evaluations for Integrative and Functional Medicine. 2nd ed. Duluth, GA: Genova Diagnostics; 2012.
4. Lemanski PE. Beyond Routine Cholesterol Testing: The Role of LDL Particle Size Assessment. https://bridgeport.instructure.com/courses/1718508/files/100458566?module_item_id=23162035. Accessed January 15, 2020.
5. Tymoczko JL, Berg JM, Stryer L. Biochemistry: A Short Course. 3rd ed. New York, NY: Freeman and Company; 2015.
6. Gropper SS, Smith JL, Carr, TP. Advanced Nutrition and Human Metabolism.7th ed. Boston, MA: Cengage Learning; 2018.
7. Ilich JZ, Kelly, OJ, Kim Y, et al. Low-grade chronic inflammation perpetuated by modern diet as a promoter of obesity and osteoporosis. Arh Hig Rada Toksikol. 2014;65(2),139-148. doi: 10.2478/10004-1254-65-2014-2541.
8. Kohlstadt I. Advancing Medicine with Food and Nutrients. 2nd London, NY: CRC Press; 2012.
9. Banjari I, Hjartaker A. Dietary sources of iron and vitamin B12: Is this the missing link in colorectal carcinogenesis?Med Hypotheses. 2018;116:105-110. doi:10.1016/j.mehy.2018.05.003.
10. Lumen M, Van Der Berg C, Geelen A, et al. Supplement use and dietary sources of folate, vitamin D, and n-3 fatty acids during preconception: The GLIMP2 Study. Nutrients. 2018;10(8):1-20. doi:10.3390/nu10080962.
-Michael McIsaac
