Ivermectin is a drug which has gained widespread attention during the COVID-19 pandemic. Since that time, ivermectin has become a polarizing topic of discussion with respect to its formulations and uses. In the following sections, I would like to consider ivermectin’s discovery, history, well-understood uses, safety profile, and emerging utility/mechanisms for viral infections.
HISTORY
Ivermectin is a compound derived from microorganisms that are, thus far, uniquely found within Japanese soil.1 Its discovery occurred during the late 1960s to early 1970s by a Japanese microbiologist/bioorganic chemist Dr. Satoshi Omura; a scientist who was seeking new sources for pharmaceuticals, inspired by the knowledge that other drugs, such as antibiotics, found their origins in nature.2 Over time, Dr. Omura and his colleagues from the Kitasato Institute (Tokyo) developed screening methodologies to identify potentially promising compounds from soil.2(1)
Dr. Omura arranged an agreement circa 1973 with Merck, a pharmaceutical research and development corporation, whereby he would send the most promising soil samples with bacteria (Streptomyces avermectiniu) to them for further development.2(1) Merck research on the soil samples was spearheaded by Dr. William Campbell, veterinary scientist and zoologist by training. Ultimately, the Merck team isolated active components produced by bacteria and named it avermectin.2(2) The original substance (avermectin) was slowly modified to become increasingly potent (25 times more potent than avermectin), and was eventually named ivermectin.2(2)
WIDELY KNOWN USES
Dr. Campbell tested ivermectin, initially, within livestock infected with various microorganisms such as parasites found in cattle, pigs, horses, dogs, and sheep.2(2) Merck discovered that ivermectin was highly effective and safe at neutralizing such parasitic infestations, thereby circumventing potential economic burdens within the livestock industry.2(2) Additional testing and research identified the utility of ivermectin against gastrointestinal roundworms, mites, lungworms, lice, and horseflies.1(14) After years of research, ivermectin was introduced as a commercial product for Animal Health in 1981.1(14)
As time passed, ivermectin was slowly considered as an intervention for human diseases to include Onchocerciasis; a condition otherwise known as river blindness, affecting billions of individuals throughout the world.2(14) Ivermectin was also found to be a safe and effective drug for Ascariasis (roundworm), Strongyloidiasis (roundworm), cutaneous larva migrans (parasitic skin infection), Gnathostomiasis (food-borne parasitic infection) and Trichuriasis (parasite found in dirt, vegetables, and fruit), scabies, (mite infestation), and Pediculosis (lice infestation).1(14) Ultimately, ivermectin evolved from a soil sample into a medicine helping billions of individuals, globally.
SAFTEY PROFIILE
Due to the effectiveness and safety of ivermectin in treating onchocerciasis and lymphatic filariasis (affecting billions, mentioned previously), its discoverers were awarded the Nobel Prize in 2015 and was officially considered an essential medicine by the World Health Organization.3 Multiple studies have explored the safety of ivermectin to include the research of Munoz et al.4 where 18 mg and 36 mg doses were used as well as the standard 0.15-0.2 mg/kg regimen in 54 healthy adult volunteers.4(1) The intent was to compare and study much larger doses to the standard dose, and record potential adverse events.
The study was a single dose, three-period, comprising 3 experimental phases of treatment with different doses of ivermectin.4(16) Each experimental period lasted from 12 h prior to drug administration to 7 days post dose followed by 14-day washout period between each dose.4(16) Group 1 weighed between 51-65 kg, group 2 weighed between 66-79 kg, while group 3 weighed equal to or over 80 kg.4(16) Vital signs (blood pressure, heart rate), hematology, blood chemistry, and urinalysis of each participant was closely monitored before, during, and after each treatment intervention. The study indicated no serious adverse events or significant differences between groups, except for non-severe headaches.4(16)
EMERGING USES FOR SARS-COV-2
Considering that SARS-CoV-2 is a virulent pathogen, which replicates aggressively and induces excessive inflammation (the latter of which can be fatal left unmitigated), it is imperative to consider all safe and effective interventions in managing such a disease.10 Thus, steps have been taken by researchers to consider and explore the utility of ivermectin for the same. Dr. Omura and colleagues conducted a comprehensive review of ivermectin and determined that the preponderance of evidence demonstrated notable reductions in mortality (death from disease) and morbidity (the severity/degree of disease) from the implementation of ivermectin.10(1) As of January 2021, 91 studies over 21 countries began conducting clinical trials with ivermectin.11
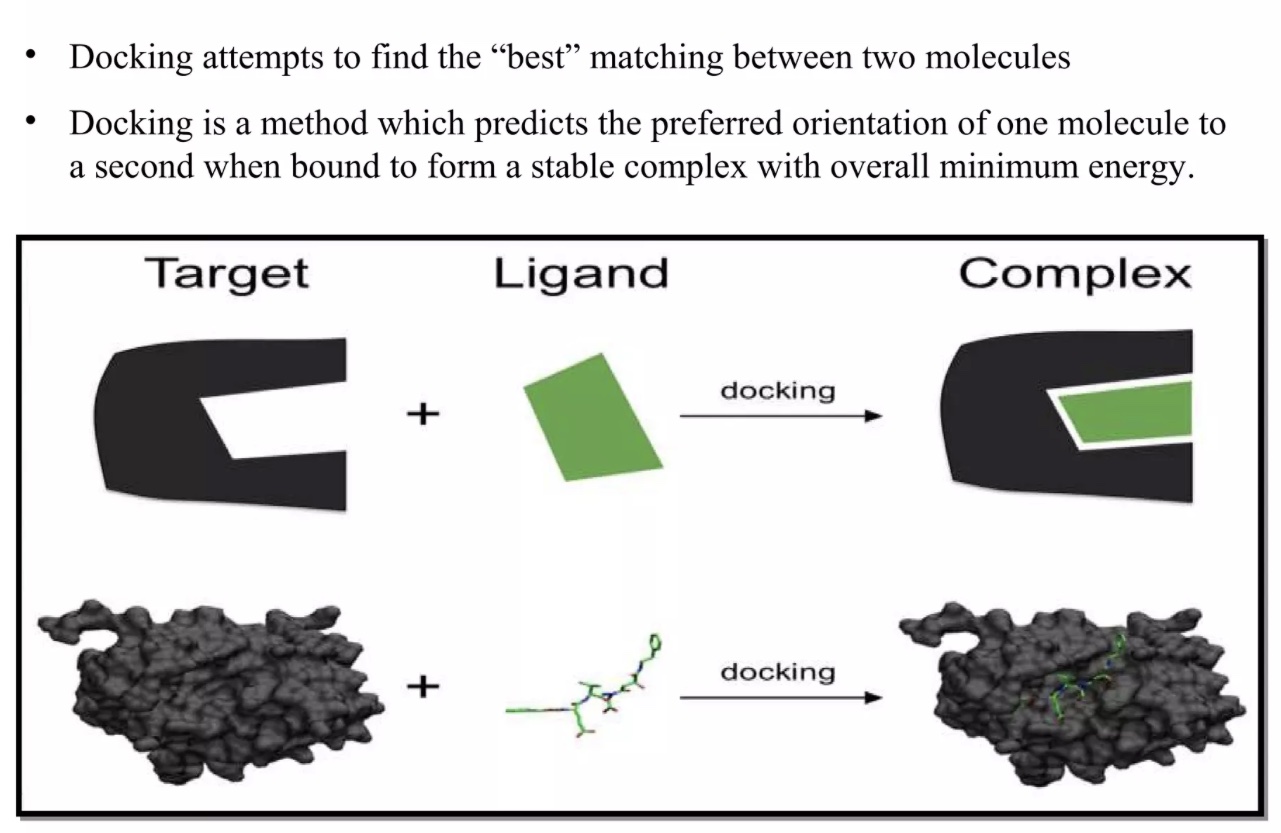
Clinical trials have now indicated that ivermectin possesses antiviral and anti-inflammatory qualities.5 Mechanistically, docking studies (molecular modeling using software to predict preferred orientation of one molecule relative to another molecule) suggested that ivermectin, compared to hundreds of other molecules, had the highest binding affinity to SARS-CoV-2 spike protein receptor binding domain (RBD-the component that interacts with host cells).3(e301),6 Additionally, ivermectin has been shown to interfere with SARS-CoV-2 structural and non-structural binding proteins (required for replication) and RNA-dependent RNA polymerase; an enzyme critical for viral replication.7,8,9 This might help explain why clinical trials are showing favorable outcomes.
 Mascolo et al.11(67) indicated that 27 clinical trials with a total of 6,612 patients (8 trials exploring prevention and 19 trials treating illness) found that ivermectin prevented infectivity to exposed individuals, reduced the need for hospitalization, accelerated recovery, and reduced mortality. As such, and due to the safety and low cost of ivermectin, said drug has been utilized in 24 countries around the world for COVID-19.11(72) Furthermore, 14 countries have implemented ivermectin, nationwide (outlined in dark green in the illustration above).11(72) It should be emphasized that prior to the COVID-19 pandemic, ivermectin was already used for over 30 years with doses that have exceeded 3.7 billion worldwide; reassuring data supporting its prior use, acceptance, and saftey.11(72)
Mascolo et al.11(67) indicated that 27 clinical trials with a total of 6,612 patients (8 trials exploring prevention and 19 trials treating illness) found that ivermectin prevented infectivity to exposed individuals, reduced the need for hospitalization, accelerated recovery, and reduced mortality. As such, and due to the safety and low cost of ivermectin, said drug has been utilized in 24 countries around the world for COVID-19.11(72) Furthermore, 14 countries have implemented ivermectin, nationwide (outlined in dark green in the illustration above).11(72) It should be emphasized that prior to the COVID-19 pandemic, ivermectin was already used for over 30 years with doses that have exceeded 3.7 billion worldwide; reassuring data supporting its prior use, acceptance, and saftey.11(72)
One particular study, in Peru, is quite intriguing. From approximately August 1/2020 to September 1/2020, ten states of Peru underwent mass ivermectin treatments led by an army effort known as Mega-Operacion Tayta (MOT).12 Within 30 days, excess all cause deaths dropped precipitously from peak deaths by an average of 74%.12(3) Nationwide, excess all cause deaths decreased 14 fold over a 16-week period up to November 17/2020 (See figure 1A above).12(3) However, November 17/2020 marked a change in Peruvian presidents, whereby the newly elected president enforced restrictions on ivermectin distribution; excess all cause deaths increased 13-fold over the next 8 weeks (see figure 1A).12(3) Other researchers noted a meta-analysis of 15 trials, which assessed a total of 2488 participants, which was found to reduce the risk of death by approximately 62% when compared to no ivermectin treatments. 5(e441)
ARGUMENTS AGAINST IVERMECTIN
Despite proposed mechanisms of action and available research, regulators of some countries claim there is insufficient evidence to accept ivermectin as a potential adjunct intervention to support the immune system during COVID-19.11(78) However, ivermectin does have a 30-year history with 3.7 billion doses demonstrating, at minimum, its robust safety profile.11(78) Regulators have also stated that existing data on ivermectin is biased (study plans and methods) and are likely insufficient to determine validity. However, a meta-analysis of 14,906 patients in 42 clinical trials using ivermectin demonstrated sufficient efficacy for the same in managing COVID-19, both in treatment and prevention.11(78, 82) Yagisawa et al.11(78) reasoned that if regulators consider such studies to be inadequate that legitimate, compelling, and cogent explanations for such determinations should be a requirement.
CONCLUSIONS
In conclusion, ivermectin is a drug which has gained widespread attention during the COVID-19 pandemic. Since that time, ivermectin has become a polarizing topic of discussion (in some countries) with respect to its formulations and uses. It is my hope that covering ivermectin’s discovery, history, well-understood uses, safety profile, and emerging utility/mechanisms for viral infections has helped view said medication in a more balanced and fair manner. It is possible that implementation of ivermectin with other antiviral/anti-inflammatory micronutrients covered previously (i.e., D3, quercetin, zinc, vitamin C, omega-3 fatty acids, black cumin seed oil, curcumin, melatonin) may have a synergistic/compounding/complementary effect; such could ultimately support the immune system during COVID-19. However, I strongly recommend a discussion between individuals and their family physicians, before considering or implementing such protocols.
Please see link on micronutrient support for COVID-19:
LINK: Covid-19, Micronutrients, and Immune Support
References
1. Crump A, Omura S. Ivermectin, ‘wonder drug’ from Japan: The human use perspective. Proc Jpn Acad Ser Phys Biol Sci. 2011;87(2):13-28. doi:10.2183/pjab.87.13.
2. Rovner S. Discovery of Ivermectin. American Chemical Society. https://www.acs.org/content/dam/acsorg/education/whatischemistry/landmarks/discovery-of-ivermectin-mectizan.pdf. Accessed November 14, 2021.
3. Kory P, Meduri GU, Varon J, et al. Review of the emerging evidence demonstrating the efficacy of ivermectin in the prophylaxis and treatment of COVID-19. Amer J Ther. 2021;28(3):e299-e318. doi:10.1097/MJT.0000000000001377.
4. Munoz J, Ballester MR, Antonijoan RM, et al. Saftey and pharmacokinetic profile of fixed-dose ivermectin with an innovative 18 mg tablet in healthy adult volunteers. PLOS Neg Trop Dis. 2018;12(1):1-16. doi:https://doi.org/10.1371/journal. pntd.0006020.
5. Bryant A, Lawrie TA, Dowswell T, et al. Ivermectin for prevention and treatment of COVID-19 infection: A systematic review, meta-analysis, and trial sequential analysis to inform clinical guidelines. Am J Ther. 2021;28(4):e434-e460. doi:10.1097/MJT.0000000000001402.
6. Monika G, Punam G, Sarbjot S, et al. An overview of molecular docking. Int J Drug Dev Res. 2010;2(2):219-231. https://www.researchgate.net/publication/279179780_An_overview_on_Molecular_Docking. Accessed November 14, 2021.
7. Lehrer S, Rheinstein PH. Ivermectin docks to the SARS- CoV-2 spike receptor-binding domain attached to ACE2. Vivo. 2020;34:3023–3026. doi:10.21873/invivo.12134.
8. Sen Gupta PS, Biswal S, Panda SK, et al. Binding mechanism and structural insights into the identified protein target of COVID-19 and importin-alpha with in-vitro effective drug ivermectin. J Biomol Struct Dyn. 2020:1–10. doi:10.1080/07391102.2020.1839564.
9. Swargiary A. Ivermectin as a promising RNA-dependent RNA polymerase inhibitor and a therapeutic drug against SARS-CoV2: Evidence from in silico studies. Res Square. 2020. Doi: 10.21203/rs.3.rs-73308/v1.
10. Mascolo S, Carleo MA, Contieri M, et al. SARS-CoV-2 and inflammatory responses: From mechanisms to the potential therapeutic use of intravenous immunoglobulin. J Med Virol. 2020;93(5):2654-2661. doi:https://doi.org/10.1002/jmv.26651.
11. Yagisawa M, Foster PJ, Hanaki H, et al. Global trends in clinical studies of ivermectin in COVID-19. Japanes J Antib. 2021;74(1):44-95. https://www.psychoactif.org/forum/uploads/documents/161/74-1_44-95.pdf. Accessed November 15, 2021.
12. Santin AD, Schiem DE, McCullough PA, et al. Ivermectin: A multifacteted drug of Nobel prize-jonoured distinction with indicated efficacy against a new global scourge, COVID-19. New Microbes New Infect. 2021;43:1-6. doi:https://doi.org/10.1016/j.nmni.2021.100924.
-Michael McIsaac







