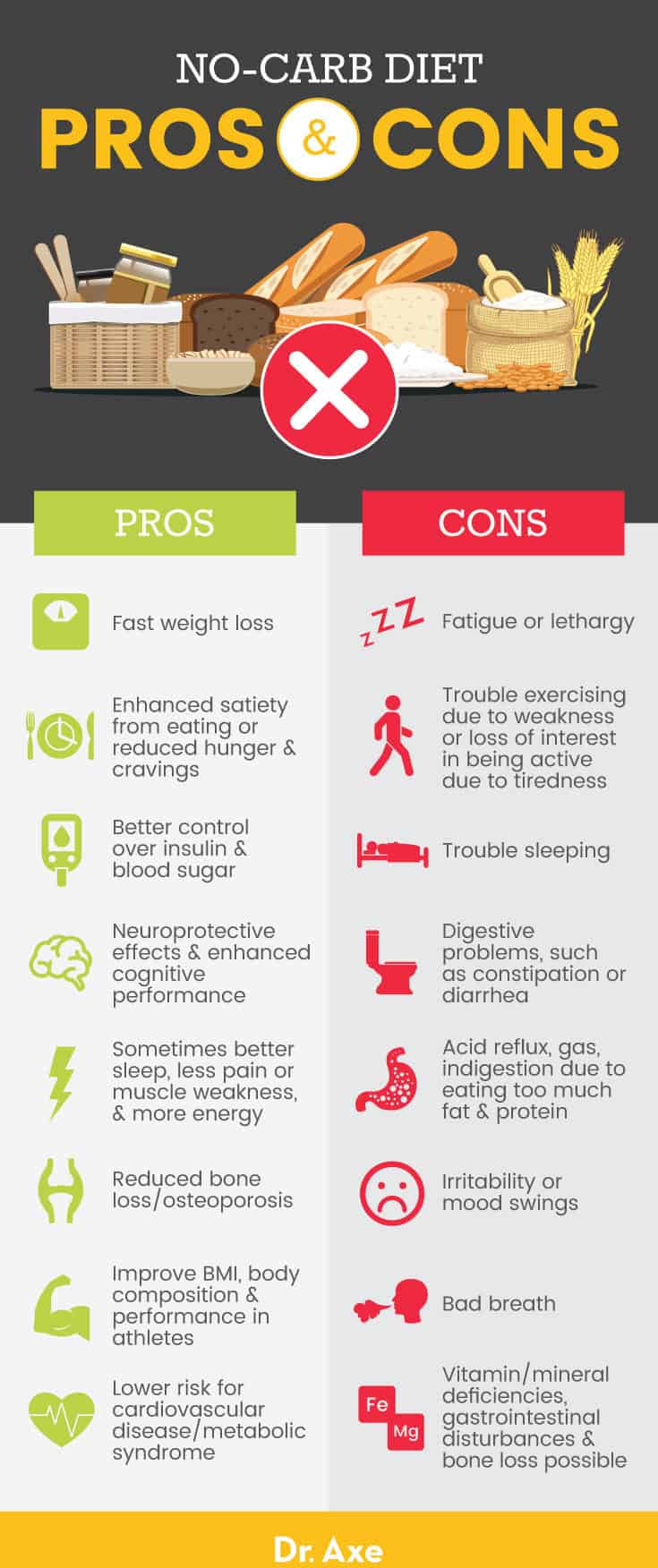
Modern Western diets tend to be rich in macronutrient sources from refined carbohydrates. In this author’s last assignment, the application of very low carbohydrate diets (VLCD) was explored as a means of treating metabolic syndrome (i.e., diabetes, poor blood lipid profiles, cardiovascular disease, obesity, and decreased insulin sensitivity) (Song, Lee, Song, Paik, & Song, 2014). Although VLCDs have been effective in treating metabolic derangement, it might be fallacious to apply said nutritional approach to all populations in a ubiquitous fashion. In the following sections, this author would like to provide examples of populations in which the VLCD approach is unnecessary and potentially deleterious, as a reminder of the biochemical and physiological variability among individuals and populations.

The people of Kitava (Papua New Guina) are largely uninfluenced by western habits and culture, and their main foods include tubers (carbohydrates), fruits (carbohydrates), vegetables (carbohydrates), in addition to fish and coconut (Lindeberg, Soè Derberg, Ahreâ, & Olsson, 2001). Evidence suggested the Kitivans derived a large portion of their daily calories from carbohydrate sources, yet were free of obesity, hypertension, cardiovascular disease and malnutrition (Lindeberg et al., 2001).
Such information seems contrary to the aforementioned benefits to VLCDs, since Western civilizations derive over 70% of their daily calories from carbohydrates, and do have metabolic syndrome (Song et al., 2014). Explanations, which help explain healthy biomarkers in the presence of high carbohydrate diets could be the types of carbohydrates consumed by Kitavans; their carbohydrate sources are void of excessive omega-6 fatty acids.
In Western society, carbohydrate sources are largely derived from processed foods, which contain high amounts of omega-6 fatty acids. The aforementioned fatty acid is strongly associated low-grade chronic inflammation/metabolic syndrome, as it is a pro-inflammatory molecule (Bengmark, 2013). Having considered carbohydrate consumption in a health-related and cultural context, the following section will consider carbohydrate manipulation and its effects on exercise performance.

Skein, Duffield, Kelly, and Marino (2012) performed a study in which they explored performance output between a pre-workout carbohydrate drink (PCD) and a pre-workout placebo carbohydrate drink (PPD). The researchers indicated that other studies examining the effects of pre-workout carbohydrate consumption allowed the participants to know which type of drink (carbohydrate or non-carbohydrate drink) they were about to consume before testing began. Skein et al. (2012) noted that prior knowledge of the drink might have affected performance output measures (i.e., participants on carbohydrate enriched drinks may have “pushed” harder that participants who consumed non-carbohydrate enriched liquids). Thus, the study design of Skein et al. (2012) acknowledged the limitations of previous research methods in two novel ways; the researchers did not tell participants the constituents of either drink, and informed them that the study was designed to assess hydration effects on performance, thereby blinding them to the true intent of the experiment.

The researchers determined that individuals who consumed PCDs performed better (i.e., higher power output during short burst sprinting) than individuals consuming PPDs. Thus, Skein et al. (2012) concluded that the study both supports the importance of PCDs for athletes, and also suggested that self-paced, intermittent-sprint intensities may be regulated based on skeletal muscle glycogen content despite the removal of a conscious regulation of pacing due to the knowledge of carbohydrate ingestion. Such evidence aligns with known concepts of substrate utilization during glycoletically demanding (i.e., sprinting, wrestling) activities; moderately intense movement that lasts between 15 seconds to 60 seconds is driven predominantly by glycolysis, which uses liver/muscle glycogen and circulating blood glucose as substrates for energy production (Kenney, Wilmore, & Costill, 2012). Thus, it is reasonable to comprehend how limiting carbohydrate consumption during moderately intense activity may decrease performance output requiring a glycoletically driven system. Having considered how low carbohydrate diets affect blood biomarkers of other cultures and performance output of athletes, the final section will consider another special population; pregnant females and caution with the implementation of a ketogenic diet.
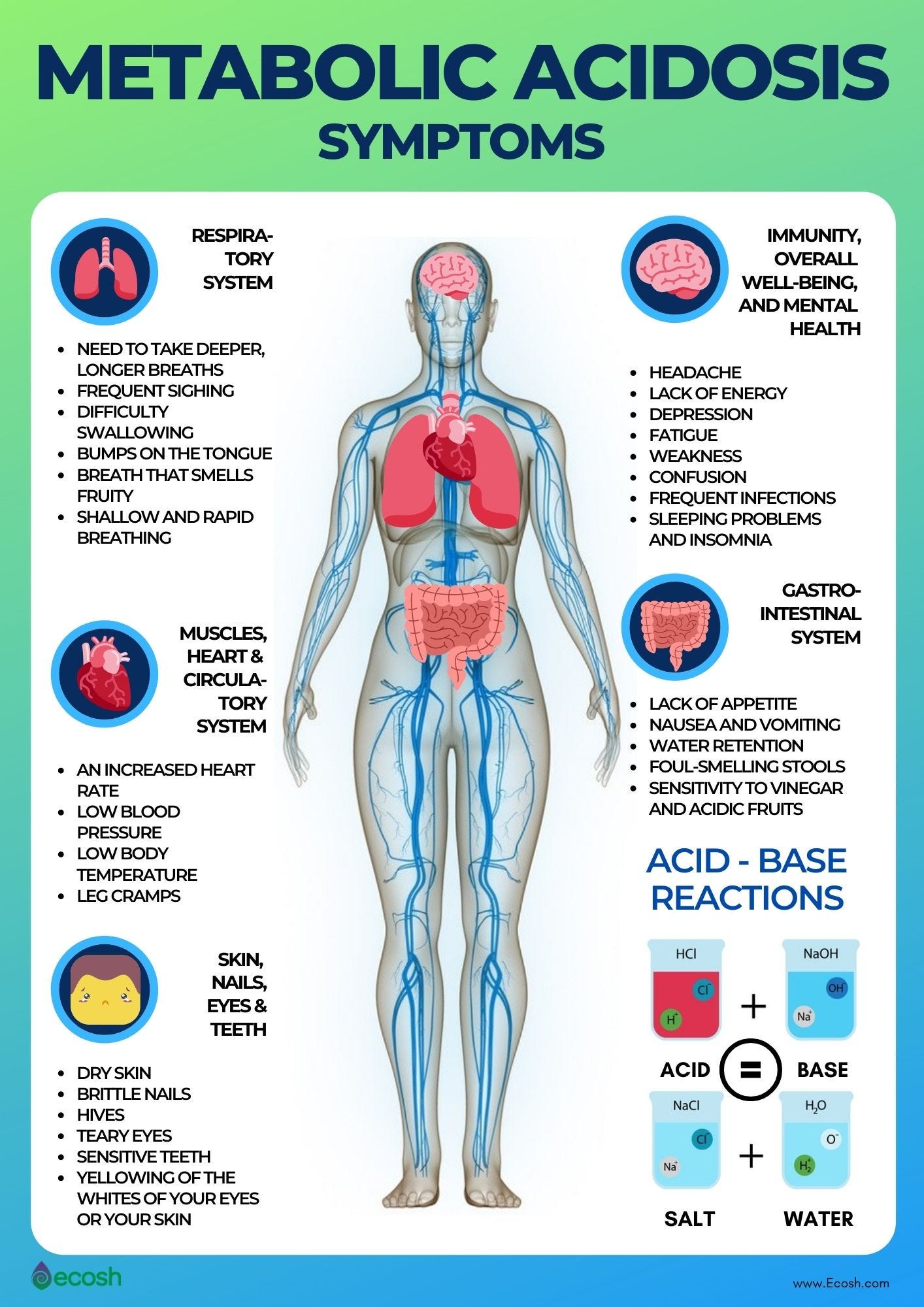
Geiger and Ekelund (2015) presented a case report in which a lactating mother was monitored while on a low-carbohydrate/ketogenic diet. A 32-year old woman admitted herself to a hospital presenting with nausea, vomiting, heart palpitations, trembling, and spasms of the extremities. Geiger and Ekelund (2015) noted that the woman reported consuming a low carbohydrate/high fat diet 10 days prior to admitting herself, with less than 20 grams of carbohydrates per day while breastfeeding her 10-month old son. Blood tests revealed the woman was experiencing acidosis, defined as excess acid due to abnormal metabolism, excessive acid intake, or renal retention or from excessive loss of bicarbonate (Metabolic Acidosis, n.d.). As a means of counteracting ketoacidosis, the woman was treated with appropriate protocols (i.e., glucose and insulin infusion) until blood biomarkers returned to normal levels. The authors noted that because lactation requires large amounts of energy substrates to provide sustenance to an infant, low-carbohydrate diets could compromise such a process, forcing gluconeogenesis (formation of glucose from non-carbohydrate sources-ketogenesis) leading to excessive proliferation/uncontrolled management of ketone bodies, and eventually ketoacidosis (Geiger & Ekelund (2015).
In conclusion, the previous examples have served as a reminder that individuals and populations exhibit biochemical and physiological variability, and it is fallacious to assume that all populations require a singular dietary approach. If one were to view nutrition as medicine, such a paradigm shift might encourage a cautious and vigilant approach when prescribing dietary interventions to individuals. Ultimately, it is essential that professionals engage in client-centered care rooted in bio-individuality. Only then can the opportunity arise for effective and precise nutritional advice.
References
Bengmark (2013). Processed foods, dysbiosis, systemic inflammation, and poor health. Current Nutrition and Food Science, 9(2), 113-143.
Geiger, L. V., & Ekelund, M. (2015). Ketoacidosis associated with low-carbohydrate diet in a non-diabetic lactating woman: a case report. Journal of Medical Case Reports, 9(1), 1-3.
Kenney, W. L., Wilmore, J. H., & Costill, D. L. (2012). Physiology of sport and exercise (5th ed.). Champaign, IL: Human Kinetics.
Lindeberg, S., Soè Derberg, S., Ahreâ, B., & Olsson, T. (2001). Large differences in serum leptin levels between nonwesternized and westernized populations: The Kitava study. Journal of Internal Medicine, 48(10), 1216-1219.
Metabolic Acidosis (n.d.). In Merriam-Webster online. Retrieved from http://www.merriam-webster.com/medical/metabolic%20acidosis
Skein, M., Duffield, R., Kelly, B. T., & Marino, F. E. (2012). The effects of carbohydrate intake and muscle glycogen content on self-paced intermittent sprint exercise despite no knowledge of carbohydrate manipulation. European Journal of Applied Physiology, 112(8), 2859-2870.
Song, S., Lee, J. E., Song, W. O., Paik, H. Y., & Song, Y. J. (2014). Carbohydrate intake and refined-grain consumption are associated with metabolic syndrome in the Korean adult population. Academy of Nutrition and Dietetics, 114(1), 54-62.
-Michael McIsaac
