
Low grade chronic inflammation (LGCI) is a condition characterized by a persistent presence of inflammatory processes, that eventually manifests as tissue damage (Nasef, Mehta, & Ferguson, 2017). LGCI is also implicated with several diseases to include autoimmune disease, cardiovascular disease, stroke, and diabetes (Kenney, Wilmore, & Costill, 2012; Nasef et al., 2017). Essentially, mitigating the inflammatory response is key in controlling the pathogenesis of said conditions, which can include the use of pantothenic acid (B5). The following will consider inflammation in greater detail, and its relationship to B5.
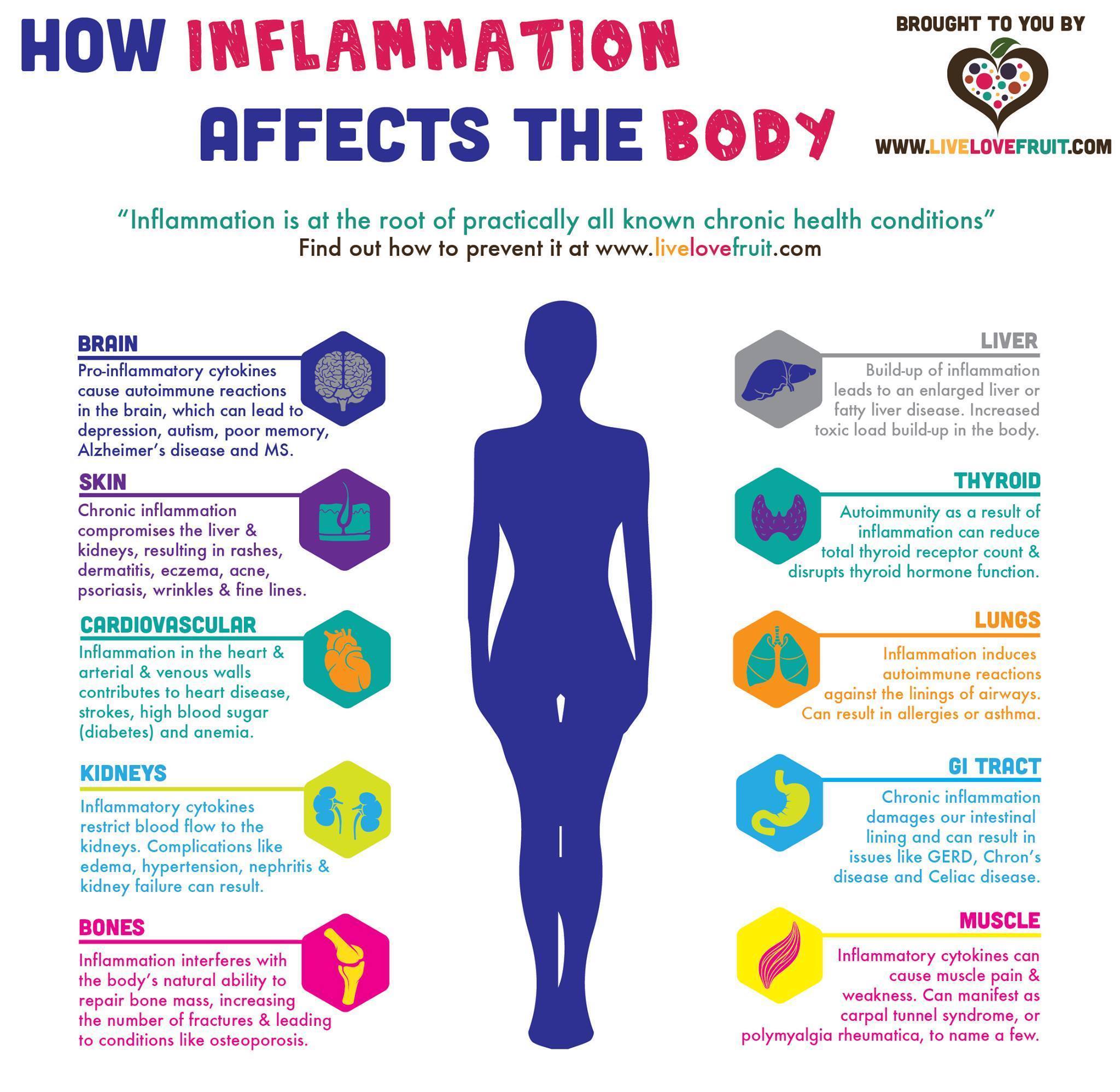
Inflammation is a normal, and necessary, physiological event that exerts a crucial role in host defense (Nasef et al., 2017). The impetus behind such a process would include exogenous factors (i.e., infection, environmental chemicals) and endogenous factors (impaired cellular metabolism, tissue injury) (Nasef et al., 2017). Ultimately, acute inflammation serves to remove the harmful stimulus, reduce/diminish further injury, and restore the function of damaged cells and tissues (Nasef et al., 2017). However, LGCI is a mismanagement of the response of macrophages, mast cells, and pro-inflammatory cytokines; cells and signaling processes central to controlling endogenous/exogenous inflammatory drivers (Nasef et al., 2017). Left unaddressed, LGCI can provide a medium for the pathogenesis of disease.
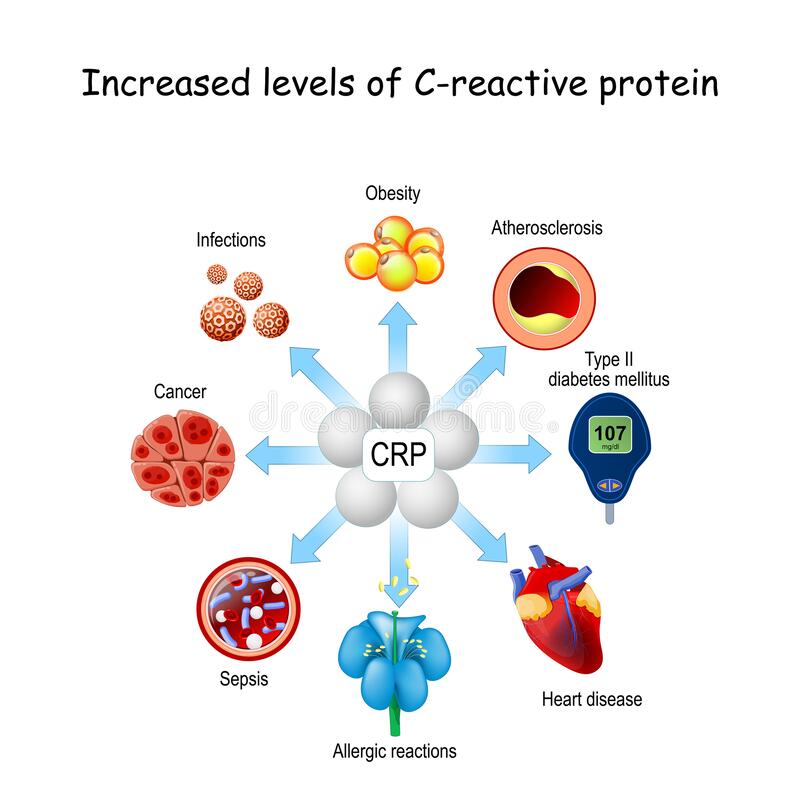
C-reactive protein (CRP) is a marker of LGCI, and its presence in sufficient levels is a means of predicting the pathogenesis of conditions such as cardiovascular disease (Jung, Kim, & Choi, 2017). As such, CRP can serve as an early warning sign to encourage deeper analysis behind rising levels in said biomarker. Interestingly, B5 is a micronutrient recognized as the precursor to coenzyme A (CoA); an essential cofactor of key biochemical reactions (i.e., energy production) (Jung et al., 2017). Of relevance is B5’s ability to help synthesize CoA, which can produce glutathione (GSH); a powerful antioxidant known to facilitate mitigation of inflammatory processes and CRP levels (Jung et al., 2017). The following will consider the anti-inflammatory effects of B5 in greater detail.
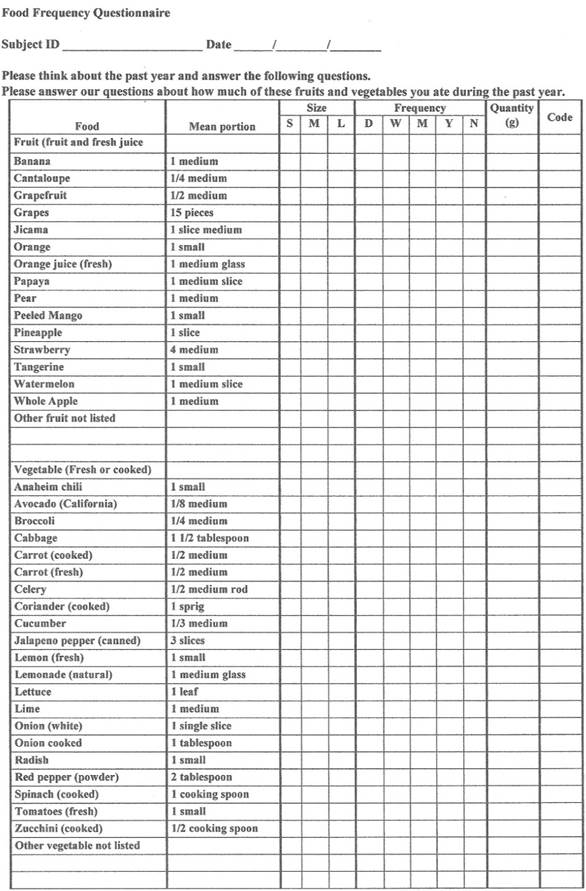
Jung et al. (2017) directed their research of B5 on reducing CRP levels and cardiovascular disease (CVD). The researchers recruited participants (212 men and 327 women) that were 40 years and older and monitored/recorded their CRP levels, among other data, between 2007 and 2013. Dietary intake and B5 consumption were gathered before the observational study began in addition to serum cytokines (TNF-αand IL-6), total and plasma cholesterol, glucose, triglycerides, blood pressure and high-density lipoproteins (HDL) (Jung et al., 2017). Such items were also monitored during the experiment in intervals between 2007-2013. B5 consumption was determined by analysis of food frequency questionnaires (FFQs); nutrient intake (in addition to B5) was ascertained using a serving size and weighted frequency/day for each food item (Jung et al., 2017). Results indicated that the average B5 intake for men was 4.0mg/day while women averaged 4.5mg/day (Jung et al., 2017). The following will consider the associations between B5 consumption and the aforementioned biomarkers monitored throughout the experiment.
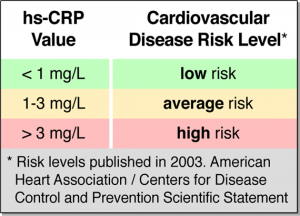
Results indicated that higher baseline B5 levels were significantly related to lower levels of CRP throughout the study. Furthermore, higher average B5 intakes (estimated B5 consumed from foods during the study) was also related to lower levels of CRP (Jung et al., 2017). The researchers hypothesized that higher B5 levels promoted CoA production, thereby increasing ATP production, which is required for the synthesis of GSH; an antioxidant that attenuates oxidative events throughout the body. It should be noted, however, that such results are associative in nature, and a direct mechanism of action was not determined by Jung et al. (2017). Furthermore, average intakes of B5 could have been limited by the structure of the FFQ; 106 items were listed with only 3 choices of serving sizes. Finally, B5 was not directly measured via blood collection and was estimated by review of the FFQ (Jung et al., 2017). Despite limitations of the observational study, it should encourage further research behind mechanism of action between B5 and inflammatory processes.
In conclusion, pantothenic acid is a micronutrient recognized as the precursor to coenzyme A (CoA); an essential cofactor of key biochemical reactions (i.e., energy production). Furthermore, Jung et al. (2017) elucidated an associative relationship between B5 levels and lower CRP, and inflammation; a progenitor of several diseased states. Such research, despite limitations of the study, should encourage individuals to consider the value of including foods rich in B5 in their daily regimen.
References
Jung, S., Kim, M. K., & Choi, B. Y. (2017). The long-term relationship between dietary pantothenic acid (vitamin B5) intake and C-reactive protein concentration in adults aged 40 years and older. Nutrition, Metabolism, and Cardiovascular Disease, 27(9), 806-816.
Kenney, W.L., Wilmore, J.H., & Costill, D.L. (2012). Physiology of sport and exercise (5th ed.). Champaign, IL: Human Kinetics.
Nasef, N. A., Mehta, S., & Ferguson, L. R. (2017). Susceptibility to chronic inflammation: An update. Archives of Toxicology, 91(3), 1131-1141.
-Michael McIsaac
