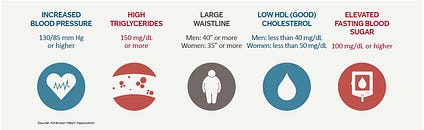
Metabolic syndrome (MS) is a condition defined by a constellation of associated risk factors that increase a person’s chances of contracting stroke, cardiovascular disease (CVD), and diabetes (Chen, Yen, Huang, Lee, Hsia, & Lin, 2012). MS affects 35.1% men and 32.6% women in the United States, and associated risk factors include high levels of blood glucose/insulin resistance (IR), large waistline/BMI, high resting blood pressure, high triglyceride levels, and low high-density lipoproteins (HDLs) (Chen et al., 2012; Teramoto et al., 2013). Traditionally, MS has been treated by allopathic medicine (i.e., blood pressure medication, statins, exogenous insulin, bariatric surgery) (Gemill, Morgan, & Borys, 2011; Kamran, Ahari, Biria, Malepour, & Heydari, 2014; Mahajan, Gupta, Gupta, & Gupta, 2010). However, the genesis of MS can be traced to lifestyle choices, which includes excessive food consumption, low physical activity, and insufficient sleep quality/duration (Bankoski et al., 2011; Saleh & Janssen, 2014; Song, Lee, Song, Paik, & Song, 2014). In the following sections, this author will explore the pathophysiology of MS in addition to interventions, which help mitigate the underlying risk factors of said disease. Such interventions will include very-low-carbohydrate ketogenic diets (VLCKDs), increased physical activity, and improved sleep hygiene.

Modern Western diets derive approximately 72% of total daily energy from cereals, dairy products, refined sugars (glucose, sucrose, and high-fructose corn syrups), refined vegetable oils (shortening, margarine, salad and cooking oils) and alcohol (Sweazea, 2014). Such overconsumption of refined carbohydrates, processed foods, and energy-dense diets has been strongly associated with obesity, cardiovascular disease, poor blood lipid profiles, decreased insulin sensitivity, and diabetes (Sweazea, 2014). Although excessive carbohydrate ingestion can elicit deleterious changes to homeostasis, the degree of IR (risk factor of MS) and type 2 diabetes (a manifestation of MS) has been mitigated and/or reversed by VLCKDs (Paoli, Rubini, Volek, & Grimaldi, 2013). As a means of appreciating the influence of nutrition on homeostasis, the following will explore VLCKDs and their affects upon the risk factors associated with MS.
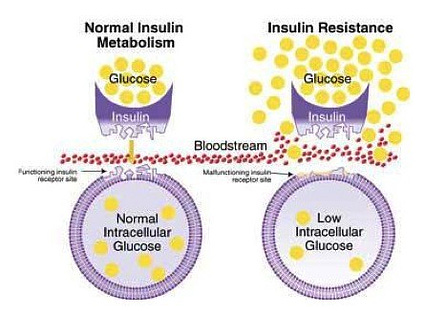
IR is a constituent of MS characterized by an aberration in glucose metabolism (Sinaiko & Caprio, 2012). Generally, an individual’s body can manage transient episodes of excess sugar consumption while maintaining optimal blood glucose levels of less than 100 mg/dl (Sanchez, Harhay, Harhay, & McElligott, 2013). However, consistent overconsumption of carbohydrates can produce IR (Funnell, 2012). Insulin secretion from the pancreas (containing beta cells that produce and release insulin) increases above normal levels (hyperinsulinemia) to counteract the target tissues’ decreased sensitivity to the aforementioned hormone (A’Damo & Caprio, 2011). IR can also manifest into a more severe condition known as hyperglycaemia, which is defined as fasting glucose levels above or equal to 100 mg/dl; despite higher levels of insulin secretion, target tissues become increasingly resistant to said hormone’s signalling (Hodgson, Govan, Ketheesan, & Morris, 2013; Sanchez et al., 2013). Although there are unfavourable affects of IR, the aforementioned risk factor of MS can be attenuated with VLCKDs.

Ingesting macronutrients that provide energy (i.e., proteins and fats) while largely devoid of sugars, allows high blood glucose concentrations (hyperglycemia) to decrease without the need for insulin. Consequently, hyperinsulinemia is also attenuated (Paoli, Rubini, Volek, & Grimaldi, 2013). Essentially, VLCKDs restrict carbohydrate ingestion to less than 50g/day encouraging a change in substrate utilization; energy production shifts from glucose sources to triglycerides (via lipolysis) in the form of ketone bodies (beta-hydroxybutyrate, acetone, acetoacetate) (Denniston, Topping, Quirk-Door, & Caret, 2016; Paoli et al., 2013). Moreover, VLCKDs not only attenuate IR. Such a protocol also helps control obesity/high BMI; another risk factor of MS.
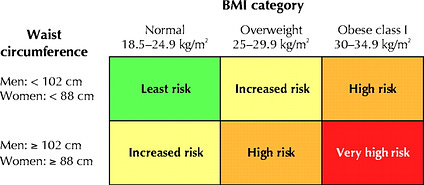
Obesity has been reaching epidemic proportions in the Westernized world, and is defined as a waist circumference of ≥102 cm for male adults, and ≥88 cm for female adults in the United States (Paoli, 2014; Sanchez et al., 2013). Physiologically, obesity can be problematic since adipose tissue is considered an active biochemical endocrine system (Sinaiko & Caprio, 2012). Sinaiko and Caprio (2012) also noted that excess accumulation of adipose tissue during the development of obesity resulted in tissue infiltration of inflammatory cells, hypoxia, and alterations in the cytokine (involved in signalling inflammatory responses) profile. Evidence has also indicated that oxidative stress and inflammation associated with obesity likely contributes to increased lipolysis and release of free fatty acids and adipokines; physiological and immunological processes known to influence IR (Sinaiko & Caprio, 2012). VLCKDs, however, can help reduce percent body fat thereby minimizing inflammatory cell signalling and oxidative stress related to the development of IR.
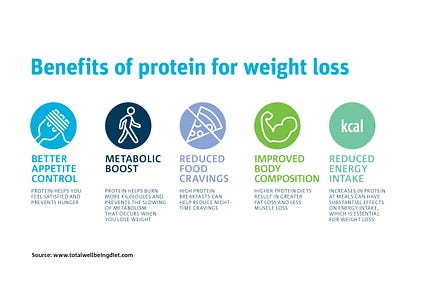
Strong supportive evidence indicates that VLCKDs are effective in weight loss therapy. However, Paoli (2014) stated that the exact mechanisms behind said affects remain unclear. Paoli (2014) did provide, from available evidence, four possible factors in which VLCKDs induced weight loss: reduction in lipogenesis and increased lipolysis, higher satiety from protein consumption, appetite suppression, increased metabolic efficiency in consuming fats, and increased metabolic costs of gluconeogenesis and the thermic effect of proteins. Additionally, VLCKDs not only help reduce bodyweight; individuals who are obese and who are also insulin resistant experienced both weight loss and improved insulin sensitivity (Paoli, 2014). Ultimately, such research indicates that VLCKDs can serve as a means to prevent and treat IR.
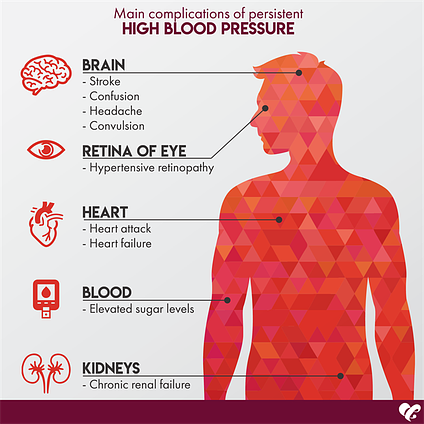
Hypertension (HT) is the third risk factor of MS, defined as systolic blood pressure ≥130 mm Hg and diastolic blood pressure ≥85 mm Hg, and has been shown to increase linearly across the whole range of waist-to-hip ratio in both men and women (Kurukulasuriya, Stas, Lastra, & Sowers, 2011; Sanchez et al., 2013). Increasing evidence has also suggested that HT might be influenced by IR and general glucose intolerance (Vasdev, Longerich, & Singal, 2002). Impaired glucose metabolism, a hallmark of IR, induces a build-up of glyceraldehyde, glyceraldehyde-3-phosphate, and dihydroxyacetone phosphate (molecules involved in carbohydrate metabolism) (Vasdev et al., 2002). Such aldehydes react unfavourably with proteins that control L-type Ca2+ channels (responsible for cardiac muscle contraction). Disruption of said calcium channels can lead to increased peripheral vascular resistance, ultimately causing HT (Vasdev et al., 2002). VLCKDs can provide a means of lowering glucose load, thereby helping reduce the concentration of aldehydes responsible for HT. VLCKDs (which can contain meats and vegetables) can also provide nutrients cited as helping reduce HT such as vitamin E, vitamin C, vitamin B6, lipoic acid, and cysteine (Vasdev et al., 2002).
Elevated triglycerides, defined as levels ≥150 mg/dl, are the fourth risk factor (hypertriglyceridemia) associated with MS (Sanchez et al., 2013). Hypertriglyceridemia (HTG) is also considered an independent risk factor for cardiovascular disease (a manifestation of MS), demonstrating its predictive value and need for monitoring (Tao et al., 2016). Additionally, HTG is strongly associated with BMI (another risk factor for MS) in addition to overall weight, waist circumference, waist/hip, and waist/height ratios (Tao et al., 2016). Thus, obesity may not only induce inflammation and IR as discussed in the previous section; it is also associated with HTG. Despite the predictive value of HTG, VLCKDs have been shown to control dyslipidemia. Gibas and Gibas (2017) cited a study whereby individuals with MS were provided a VLCKD over 56 weeks. Among several biomarkers that were tracked, triglyceride levels were dramatically reduced (41% decrease compared to pre-test values) (Gibas & Gibas, 2017). Such evidence helps support the role and influence of VLCKDs in treating HTG. Having considered HTG and its relationship to MS, the following will explore the final risk factor (low HDLs) and its affect upon the aforementioned disease.

Low HDLs represent the fifth, and final, risk factor for MS and is defined as HDL-C <40 mg/dl in men or <50 mg/dl in women (Sanchez et al., 2013). Zheng and Aikawa (2012) stated that every 1-mg/dl decrease in HDL-C concentration was associated with a 2% to 3% increased risk of cardiovascular disease, indicating the strong influence of HDL upon cardiovascular function. Higher HDLs may induce protective effects against CVD via multiple mechanisms of action. One hypothesis suggests that HDLs carry plaque-forming lipids to the liver, helping control atherosclerotic buildup (Zheng & Aikawa, 2012). HDLs also exhibit anti-inflammatory, antithrombotic, and anti-oxidant effects, all of which are thought to protect endothelial function (Zheng & Aikawa, 2012).
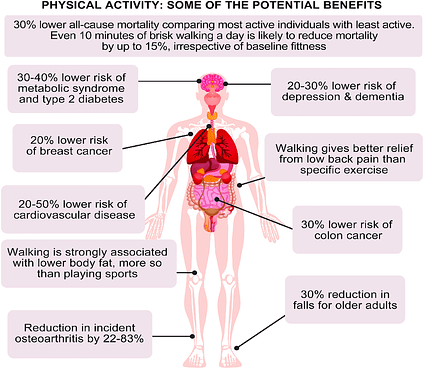
Longo-Mbenza et al. (2011) stated that MS, among other non-communicable diseases, were leading causes of mortality worldwide. Most relevantly, said diseases were associated with sedentary behaviours, defined as time spent sitting or lying during waking hours (Saleh & Janssen, 2014). Such inactivity in North America was further established by the work of Matthews et al. (2008); 6,329 participants in the researchers’ study between 2003 and 2004 were found to have spent 7.7 hours a day (average) between sedentary and seated activities. Longo-Mbenza et al. (2011) also noted that among lifestyle choices that included healthier nutrition, physical activity was another means of reducing risk factors associated with MS. The researchers stated that physical activity was shown to reduce fat mass, hypertension, triglycerides, blood glucose, and increase HDL cholesterol (Mbenza et al., 2011). Essentially, all 5 risk factors associated with MS were improved by including physical activity among individuals with MS. For example, IR occurs from a reduction in insulin receptors (GLUT-4 glucose transporters) along cell membranes (Kenney, Wilmore, & Costill, 2012). Muscle contractions that occur during exercise, however, produce insulin-like activity via increasing membrane permeability. Thus, exercise provides a second means of controlling risk factors (like hyperglycaemia) in conjunction to a VLCKD. Having outlined the relevance of VLCKDs, exercise, and their affects upon MS, the following will consider the third, and final method of controlling MS via enhanced sleep hygiene.
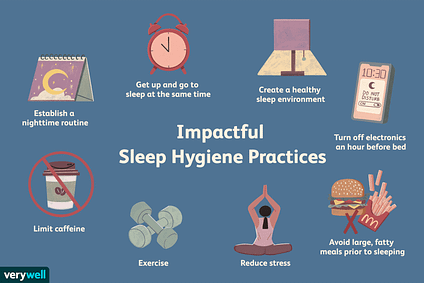
As discussed in previous sections, excessive carbohydrate consumption and sedentary behaviour has been linked to the development and exacerbation of MS. A third, and often underappreciated, constituent related to MS includes insufficient sleep, defined as less than 7 hours a night (Saleh & Janssen, 2014). Adequate sleep quality and duration allows for normal functioning of daily hormonal and metabolic processes, including the regulation of appetite (Hung, Yang, Ou, Wu, Lu, & Chang, 2013). Insufficient sleep duration/quality has also been associated with IR, diabetes, obesity, cardiovascular disease, cognitive/emotional/social impairments, and all-cause mortality (Hung et al., 2013; Tangler, Stanko, & Forbey, 2017). Despite data demonstrating the ill affects of poor sleep, the Center for Disease Control stated that over 35% of U.S. adults sleep less than 7 hours a night, 37.9% reported falling asleep unintentionally, and 4.5% reported falling asleep while driving (Tangler et al., 2017). Thus, consistent sleep quality and duration is essential to avoid exacerbation of MS, in addition to maintaining overall health and well-being.

One practical method of improving sleep quality and duration is through controlling artificial light exposure (particularly from flat screens, tablets, and smartphones) during the evening/near bedtime hours. Such devices emit a wavelength of <550 nm, commonly known as blue light (Burkhart & Phelps, 2009). Blue light can become problematic since it stimulates the photosensitive fibres with the eyes, hindering the release of melatonin; a hormone secreted from the pineal gland, which is responsible for inducing sleepiness and regulating overall wake and sleep cycles (Burkhart & Phelps, 2009). Although abstinence from blue light emitting devices in the evening is the ultimate solution to enhancing melatonin production, such an approach would likely be an unattractive choice for individuals. Providing an alternative that allows one to engage in continued use of such devices may be a more reasonable and sustainable option. Such an alternative would include the implementation of glasses with lenses that have the capacity to filter out blue light wavelengths. Amber-coloured lenses would elicit such an outcome (Burkhart & Phelps, 2009).

Burkhart and Phelps (2009) conducted a study whereby 20 participants (average age 34 years) were randomly assigned to use amber glasses or placebo glasses. All individuals had sleep-onset and mid sleep insomnia. Additionally, both groups were unaware of the amber lens hypothesis (Burkhart & Phelps, 2009). Participants were instructed to wear glasses 3 hours before bedtime and use a sleep diary indicating sleep quality on a scale of 0-10; 0 indicated poor sleep while a score of 10 indicated very good sleep (Burkhart & Phelps, 2009). Individuals implementing amber lenses noted statistically significant improvements in sleep and mood compared to controls (Burkhart & Phelps, 2009). Considering cost-effectiveness and ease-of-use of amber lenses, such an intervention provides a reasonable option to attenuate insufficient sleep and help reduce the onset and manifestation of MS.
MS shares an intimate relationship with food, physical activity, and sleep. When abused and mismanaged, excessive carbohydrate/processed food consumption, inactivity, and poor sleep can manifest and exacerbate risk factors associated with MS. Conversely, the coalescence of VLCKDs, increased physical activity, and improved sleep duration can provide the opportunity to prevent and/or completely mitigate risk factors associated with MS. In conclusion, awareness of such evidence-based research should enable individuals to reach homeostasis. Ultimately, and most relevantly, such knowledge should provide hope, incentive, and guidance to those who wish to become liberated from MS.
References
A’Damo, E., & Caprio, S. (2011). Type 2 diabetes in youth: Epidemiology and pathophysiology. Diabetes Care, 34(2), 5161-5165.
Bankoski, A., Harris, T. B., McClain, J. J., Brychta, R. J., Caserotti, P., Chen, K. Y., … Koster, A. (2011). Sedentary activity associated with metabolic syndrome independent of physical activity. Diabetes Care, 34(2), 497-503.
Chen, S. J., Yen, C. H., Huang, Y. C., Lee, B. J., Hsia, S., & Lin, P. T. (2012). Relationships between inflammation, adiponectin, and oxidative stress in metabolic syndrome. Plos One, 7(9), e45693.
Dashti, H. M., Matthews, T. C., Hussein, T., Ashfar, S. K., Behbahani, A., Khoursheed, M. A., … Al-Zaid, N. S. (2004). Long-term effects of a ketogenic diet in obese patients. Clinical Cardiology, 9(3), 200-205.
Denniston, K. J., Topping, J. J., Quirk-Door, D. R., & Caret, R. L. (2016). General, organic, and biochemistry (9th ed.). New York, NY: McGraw-Hill Education.
Funnell, M. M. (2012). Understanding insulin resistance. Nursing, 42(3), 62.
Gemill, J., Morgan, D. L., & Borys, D. (2011). 71 outcomes and clinical effects from the pediatric ingestion of the cholesterol medication fenofibrate. Annals of Emergency Medicine, 58(4), S201.
Gibas, M. K., & Gibas, K. J. (2017). Induced and controlled dietary ketosis as a regulator of obesity and metabolic syndrome pathologies. Diabetes and Metabolic Syndrome: Clinical Research and Reviews. Retrieved from http://ac.els-cdn.com.libproxy.bridgeport.edu/S1871402116303137/1-s2.0-S1871402116303137-main.pdf?_tid=0a43dafa-7a10-11e7-8507-00000aacb35e&acdnat=1501959677_526501163d358411bcb73f3122dacc66
Hodgson, K., Govan, B., Ketheesan, N., & Morris, J. (2013). Dietary composition of carbohydrates contributes to the development of experimental type 2 diabetes. Endocrine, 43(2), 447-451.
Huang, H. C., Yang, Y. C., Ou, H. Y., Wu, J. S., Lu, F. H., & Chang, J. (2013). The association between self-reported sleep quality and metabolic syndrome. Plos One, 8(1), e54304.
Kamran, A., Ahari, S. S., Biria, M., Malepour, A., & Heydari, H. (2014). Determinants of patient’s adherence to hypertension medications: Application of health belief model among rural patients. Annals of Medical & Health Sciences Research, 4(6), 922-927.
Kenney, W. L., Wilmore, J. H., & Costill, D. L. (2012). Physiology of sport and exercise (5th ed.). Champaign, IL: Human Kinetics.
Kurukulasuriya, L. R., Stas, S., Lastra, G., & Sowers, J. R. (2011). Hypertension in obesity. Medical Clinics of North America, 95(5), 903-917.
Longo-Mbenza, B., Mvindu, H. N., On’Kin, J. B. K., Bikuku, N., Phanzu, B. K., Okwe, A. N., & Kabangu, N. (2011). The deleterious effects of physical inactivity on elements of metabolic syndrome in Central Africans at high cardiovascular risk. Diabetes & Metabolic Syndrome: Clinical Research & Reviews, 5(1), 1-6.
Mahajan, R., Gupta, A., Gupta, R., S., & Gupta, K. (2010). Efficacy, safety, and cost-effectiveness of insulin sensitizers as add-on therapy in metabolic syndrome in patients with secondary sulfonylurea failure: A comparative study. Journal of Pharmacology and Pharmacotherapeutics, 1(2), 82-86.
Matthews, C. E., Chen, K. Y., Freedson, P. S., Buchowski, M. S., Beech, B. M., Pate, R. R., & Troiano, R. P. (2008). Amount of time spent in sedentary behaviors in the United States, 2003-2004. American Journal of Epidemiology, 167(7), 875-881.
Na, K. S., Kim, W. H., Jung, H., Y., Ryu, S., G., Min, K. J., Park, K. C., … Kim, C. E. (2012). Relationship between inflammation and metabolic syndrome following treatment with paliperidone for schizophrenia. Progress in Neuro-Psycopharmacology & Biological Psychiatry, 39(2), 295-300.
Paoli, A. (2006). Ketogenic diet for obesity: Friend or foe? International Journal of Environmental Research and Public Health, 11(2), 2092-2107.
Paoli, A., Rubini, A., Volek, J. S., & Grimaldi, K. A. (2013). Beyond weight loss: A review of the therapeutic uses of very low-carbohydrate (ketogenic) diets. European Journal of Clinical Nutrition. 67, 789-796.
Saleh, D., & Janssen, I. (2014). Interrelationships among sedentary time, sleep, duration, and the metabolic syndrome in adults. BMC Public health, 14(1), 666.
Sanchez, H., Harhay, M. O., Harhay, M. M., & McElligott, S. (2013). Prevalence and trends of metabolic syndrome in the adult U.S. population, 1999-2010. Journal of the American College of Cardiology, 62(8), 697-703.
Sinaiko, A. R., & Caprio, S. (2012). Insulin Resistance. The Journal of Pediatrics, 161(1), 11-15.
Sweazea, K. L. (2014). Compounding evidence implicating Western diets in the development of metabolic syndrome. Acta Physiologica, 211(3), 471-473.
Tangler, M. J., Stanko, K. A., & Forbey, J. D. (2017). Predicting sleep hygiene: A reasoned approach. Journal of Applied Social Psychology, 47(1), 3-12.
Tao, L. X., Yang, K., Liu, X. T., Cao, K., Zhu, H. P., Luo, Y.X., … Guo, X. H. (2016). Longitudinal associations between triglycerides and metabolic syndrome components in a Beijing adult population. International Journal of Medical Sciences, 13(6), 445-450.
Vasdev, S., Longerich, L., & Singal, P. (2002). Nutrition and hypertension. Nutrition Research, 22(1-2), 111-123.
Westman, E. C., Yancy, W. S., Olsen, M. K., Dudley, T., & Guyton, J. R. (2006). Effect of a low-carbohydrate, ketogenic diet program compared to a low-fat diet on fasting protein subclasses. International Journal of Cardiology, 110(2), 212-216.
Zheng, C., & Aikawa, M. (2012). High-density lipoproteins. Journal of the American College of Cardiology, 60(23), 2380-2383.
-Michael McIsaac