The liver is the largest organ in the body providing several vital functions such as storing glycogen, copper, iron, triglycerides, and lipid soluble vitamins (Reisner & Reisner, 2017). The liver is also responsible for synthesizing certain proteins such as albumin, which facilitates coagulation and inflammation, in addition to binding proteins for storage of substances (Reisner & Reisner, 2017). Finally, the liver is responsible for energy production by metabolizing glucose and fatty acids, and is responsible for catabolizing substances such as hormones and exogenous toxic agents (Reisner & Reisner, 2017). Due to the wide array of vital functions of the liver, damage inflicted upon such an organ can threaten homeostasis. As a means of appreciating liver function, the following will explore non-alcoholic fatty liver disease (NAFLD), its etiology, pathophysiology, and treatment methods.
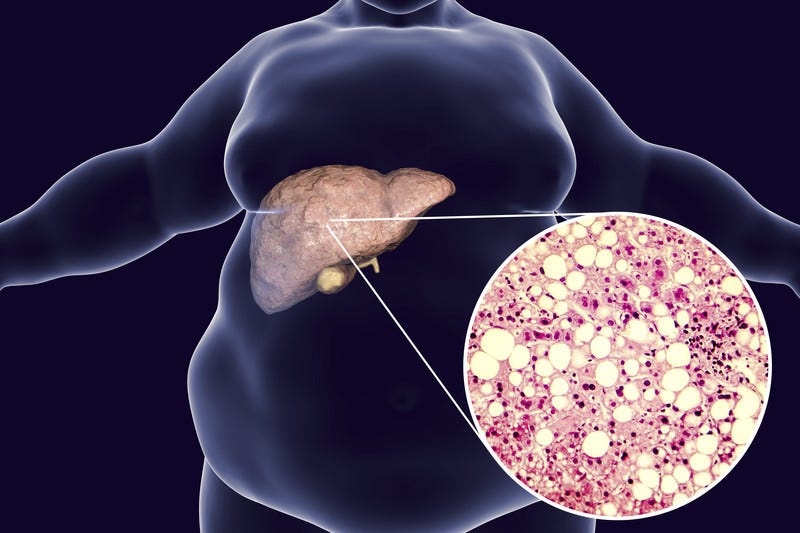
Individuals who are obese and/or have diabetes tend to accumulate fat in the liver. The individual also tends to have higher than normal blood glucose concentrations in the blood, which is related to obesity, type 2 diabetes, and insulin resistance (Reisner & Reisner, 2017). The co-existence of said factors is known as metabolic syndrome, and increased fat infiltration within the liver tends to inflict damage upon the hepatic cells. Specifically, fatty accumulation/infiltration causes cell necrosis and scarring of the liver, ultimately affecting function, and eventually threatening life (Reisner & Reisner, 2017). NAFLD and its associated complications are becoming more frequent as obesity and diabetes continues to propagate in modern Westernized societies. However, there are interventions that show promise in managing NAFLD.
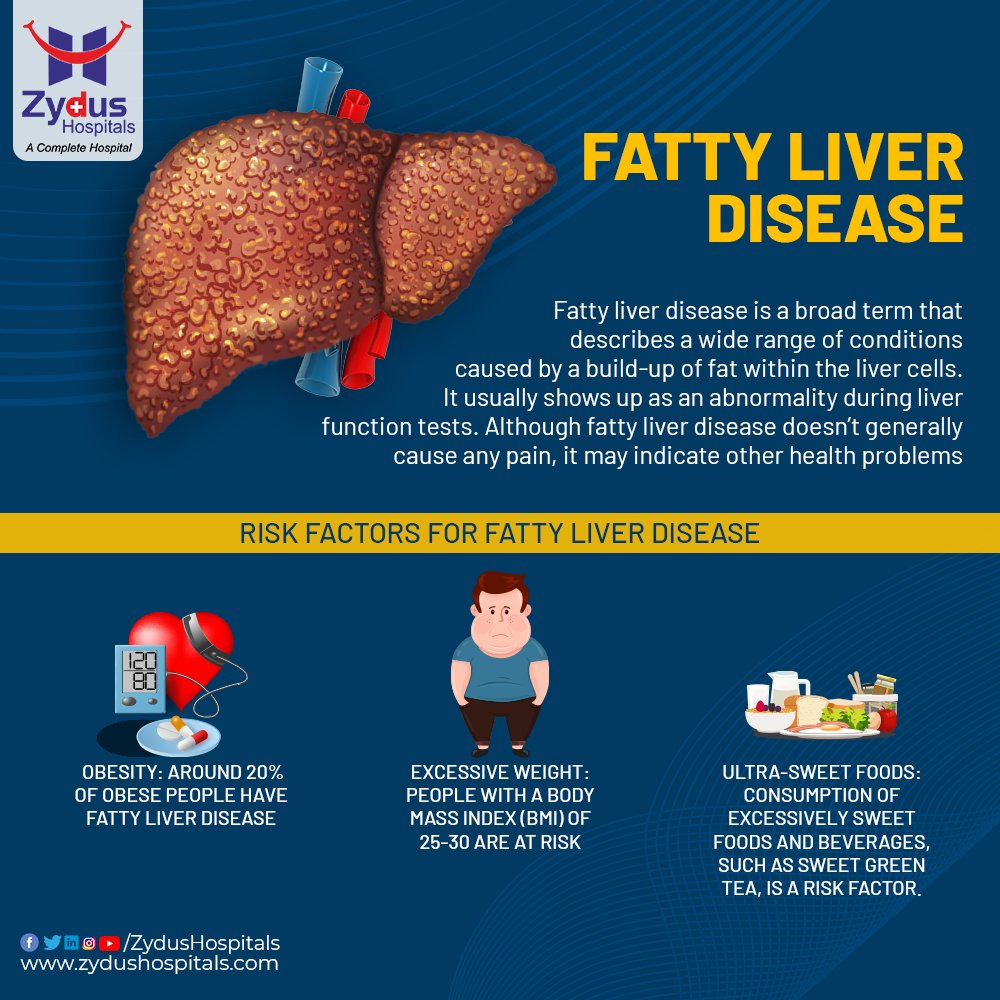
Currently, advice on nutrition, weight loss, and the energetic management of metabolic syndrome constitutes the bulk of treatment for NAFLD (Masterjohn, Plevris, & Hayes, 2010). Interestingly, studies of the dietary habits of NAFLD patients revealed that they consumed less oily fish, but doubled the quantity of soft drinks and 27% more meat compared with the general population (Masterjohn et al., 2010). Such dietary proclivities are associated with an increased risk of NAFLD independent of traditional risk factors (Masterjohn et al., 2010). Having considered poor nutrition and its affects upon liver function, Masterjohn et al. (2010) suggested promising data from both animal and human trials on the use of omega-3s in NAFLD.

Omega-3 (N-3) fatty acids are polyunsaturated fatty acids (PUFAs), and cannot be synthesized in the body making them an essential fatty acid (Masterjohn et al., 2010). Large quantities of N-3 are found naturally in fish oil, flaxseed, and some nuts. They derive from alpha linolenic acid (ALA) but mainly occur as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), which are both anti-inflammatory in nature (Masterjohn et al., 2010). However, N-3 has a counterpart; Omega-6 (N-6) fatty acids, which are pro-inflammatory and derived from arachadonic acid. The following section will explore the symbiotic relationship of both polyunsaturated fatty acids.
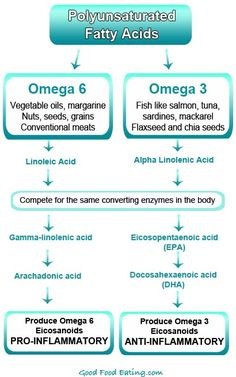
N-6 and N-3 fatty acids (PUFAs) are competitively metabolized by the same pathway. It has also been suggested that the ratio of N-6 to N-3 should be approximately 3:1, respectively. However, Masterjohn et al. (2010) stated that the modern Western diet is rich in N-6 foods, making the ratios as high as 15:1, which is correlated to steatosis (accumulation of liver fat), increased oxidative stress and non-alcoholic steatohepatitis (liver inflammation and damage from fat build-up).

N-3 fatty acids are key regulators of liver gene transcription, particularly with regards to peroxisome proliferator-activated receptor alpha (PPARα) and sterol regulatory element binding protein-1 (SREBT-1) (Masterjohn et al., 2010). Expression of PPARα is essential because it helps reduce plasma lipids and increases mitochondrial beta-oxidation (generating energy from fat). Furthermore, Masterjohn et al. (2010) indicated that increasing PPARα could prevent steatohepatitis and reverse the disease, in addition to providing an anti-inflammatory affect by suppressing tumor necrosis factor alpha (a cell-signalling protein involved in systemic inflammation) and interleukin-6 (a cell-signalling protein, which signals an immune response).

SREBT-1 plays a role in insulin resistance and is also a key regulator of fatty acid synthesis (Masterjohn et al., 2010). High insulin and glucose levels stimulate synthesis of SREBT-1, which causes it to initiate lipogenesis (synthesis of fatty acids) and glycolysis. Thus, overproduction of SREBT-1 results in the deposition and accumulation of triglycerides in the liver (Masterjohn et al., 2010). Omega-3s reduce the amount of mature (it goes through 2 steps before it is activated) SREBP-1 available in the nucleus. Such a process inhibits SREBP-1 from initiating lipogenesis.

In conclusion, NAFLD is becoming more common and proliferating worldwide. However, solutions do exist that can manage and/or mitigate the disease; N-3 fatty acids have been proposed as a nutritional intervention that has been shown to influence hepatic gene expression of substances responsible for fat accumulation and deposition within the liver. Ultimately, such information regarding N-3 fatty acids shows promise as a method of improving hepatic function and health.
References
Masterjohn, G. E., Plevris, J. N., & Hayes, P. C. (2010). Review Article: Omega-3 fatty acids- A promising therapy for non-alcoholic fatty liver disease. Alimentary Pharmacology & Therapeutics, 31(7), 679-692.
Reisner, E. G., & Reisner, H. M. (2017). An introduction to human disease: Pathology and pathophysiology correlations (10th ed.). Burlington, MA: Jones & Bartlett Learning.
-Michael McIsaac
