
Cancer is a leading cause of disease and mortality accounting for more than 8.8 million deaths in 2015, worldwide.1 Gliomas, an often-malignant type of brain cancer, are a frequently diagnosed form of tumor with an estimated 23,800 new cases identified for 2017.1(136) As such, it is imperative to develop interventions that manage and/or prevent the formation and propagation of such a cancer. The following will consider the pathophysiology of gliomas and the utility of ketogenic diets in facilitating the management of said disease.
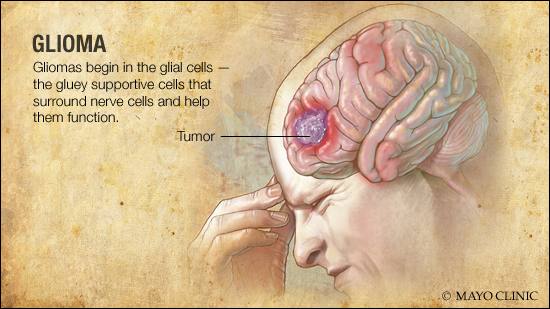
Gliomas can be characterized as neuroepithelial (a component of nerves) tumors that emanate from supporting glial cells (provide support and insulation of nerves) within the central nervous system (CNS).1(136) Glial tumors contain predominantly astrocytomas (cancers formed from glial cells) and oligodendrogliomas (gliomas formed from pre-cursor glial cells) that can be identified using molecular genetic parameters and histological methods.1(136) Isocitrate dehydrogenase (an enzyme found within the energy-producing pathways of the mitochondria) can display a mutation, which is a frequent finding in grade II and III infiltrating gliomas. Such a characteristic improves histological diagnostic accuracy enabling a favorable prognostic implication for all glial tumors according to Poff et al.1(136) Having considered basic features of gliomas, the following will explore the unique metabolic characteristics of the same in greater detail.
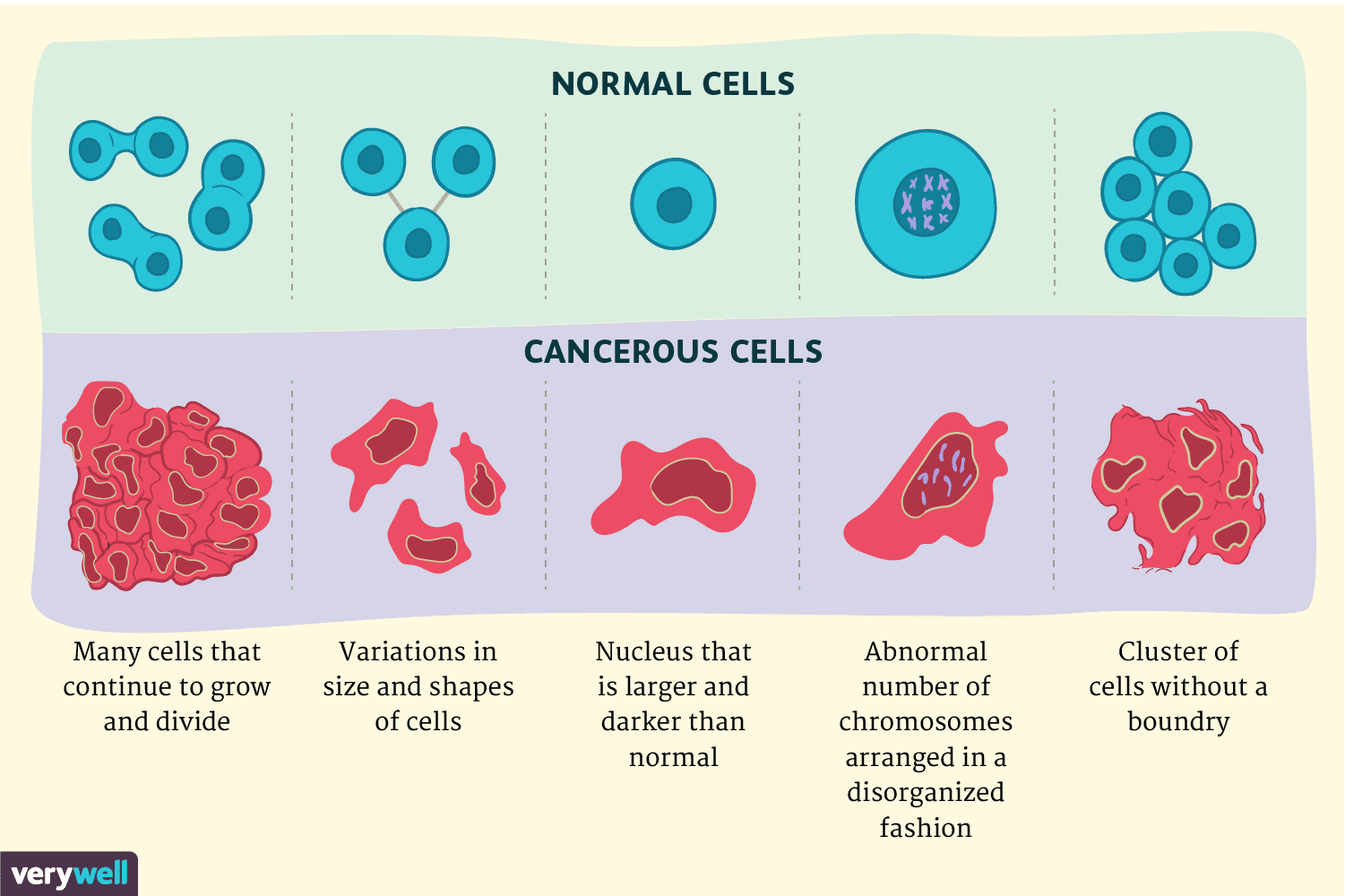
Poff et al1(136) stated that tumors exhibit a unique metabolic proclivity characterized by high rates of aerobic glycolysis in the presence of oxygen. Following glycolysis, pyruvate is primarily converted to lactate despite availability of oxygen. Other characteristics include genetic mutations, tumor hypoxia, and mitochondrial abnormalities within gliomas.1(136)Such tumor growth is a consuming process with regards to energy and resource demands. Thus, cell metabolism experiences notable alterations during tumor growth and progression inducing several growth-promoting effects: ATP despite hypoxia, a lowered cell pH (high acidity), and increased access to carbon sources for biomass production. Ultimately, such aberrations in cell metabolism causes some cancers to induce unregulated glucose energy pathways that support tumor growth.1(136) The following will consider ketogenic diets (KDs), physiological implications, and mechanisms behind down-regulating cancer growth.
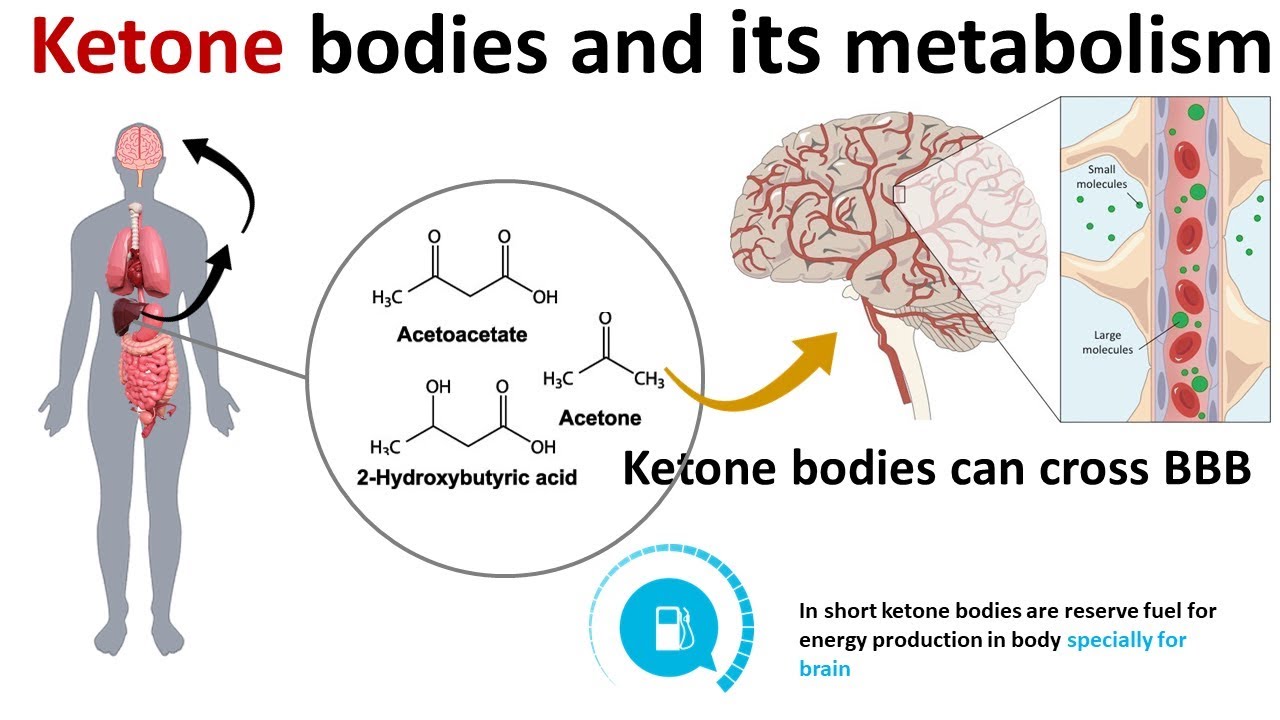
Ketones are molecules/energy metabolites that are produced within the body during periods of fasting, starvation, prolonged exercise, and/or during the consumption of a KD.1(137) KDs are generally characterized by a high fat, moderate protein, and very low carbohydrate (<50 grams/day) diet in which ketone bodies (acetone, acetoacetate, beta hydroxybutyrate) are synthesized as a dietary consequence.1(137) The KD induces a metabolic state of starvation, producing and directing a shift towards fat metabolism, while carbohydrate restriction keeps insulin and glucose levels stable and low, activating gluconeogenesis (production of glucose form non carbohydrate sources) and forcing acetyl CoA production (substrate which enters the mitochondria to make energy/ATP) via fatty acid beta-oxidation within the liver.1(137) Ultimately, KDs are thought to supress tumor growth by: reduction of glucose and insulin, modulation of oxidative stress, and reduction of inflammation, among others.1(138) The following will explore the same in greater detail.

Ketosis, a state of producing ketones, tends to supress the frequency and magnitude of insulin and glucose signaling placing a direct impact upon the growth of some cancer cells. Since such cells use glucose as a primary pathway, ketosis blunts the availability of glucose as a primary energy substrate for the mitochondria via reduction of starchy carbohydrates, and refined sugars.1(138) Such assertions are supported by research indicating hyperglycemia (high blood glucose) increases tumor growth rate in animals, and in humans.1(138)Furthermore, Poff et al1(138) cited research supporting that increased blood glucose was directly correlated to glioma growth in vivo (within an organism). The presence of glucose causes glucose transporters (GLUT) to up-regulate in cell membranes. However, such transporters are consistently denser in cancer cells. Insulin and insulin receptors are also routinely higher in number and activity in cancer cells. Such presence is problematic as insulin and insulin receptors facilitate entry of glucose, in addition to promoting cancerous cell proliferation.1(138)
KDs also help control oxidative stress; a destructive process that is up-regulated in cancerous cells.1(139) Uncontrolled oxidative stress produced from reactive oxygen species (ROS) is problematic due to the tendency for ROS to provide a growth advantage while simultaneously facilitating tumor growth and progression.1(139) Although ROS production is a normal by-product of cellular metabolism, which is increased in cancerous cells, chronic inflammation from sustained infection, hyperglycemia, or exposure to chemicals like tobacco, also provides a significant source of ROS production.1(139) Ultimately, such factors further compound an already inherent problem within a tumor cell. Ketosis is promising as it lowers basal oxidative stress levels within tumors while enhancing oxidative stress-induced damage in response to chemotherapy and radiation treatments.1(139) Furthermore, in healthy tissues, ketosis achieves lower oxidative stress by decreasing ROS production and enhancing endogenous antioxidant capacity, in a simultaneous fashion.1(139)
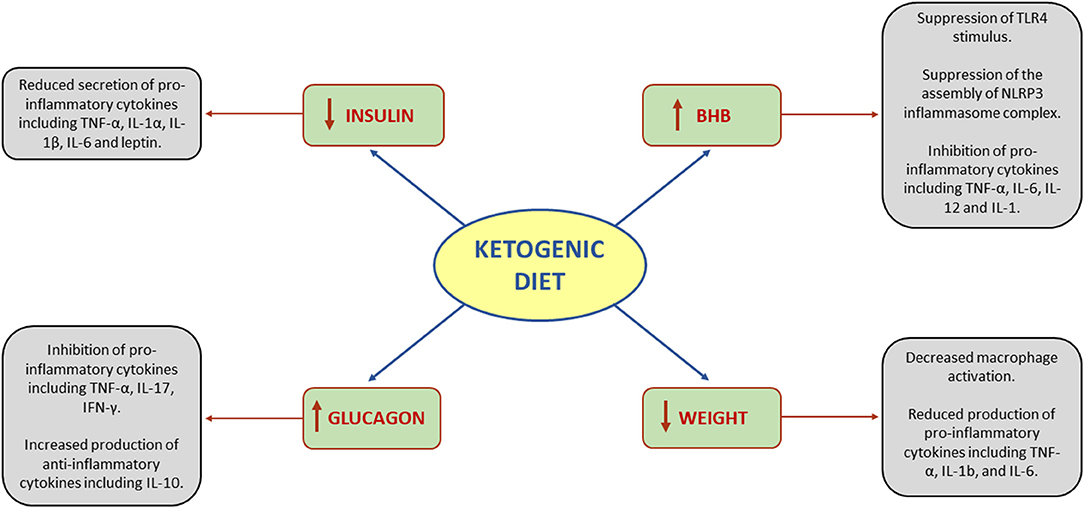
Systemic inflammation negatively impacts cancer patient health and outcomes independent of
tumor type and stage.1(139)Inflammation can emanate from acute-phase response by the liver in response to tumor, while the tumor itself can secrete inflammatory cytokines (TNF-α, IL-6, and IL-1β).1(139) Increased inflammation also tends to initiate catabolism, decrease appetite, and inhibits synthesis signaling in the skeletal muscle. Such processes contribute and leads to cancer-induced wasting.1(139) Poff et al1(139) noted that a significant contributor to inflammation is the NLRP3 inflammasome (part of the innate immune system activated by pathogens or injury). Such an inflammasome and its up-regulated activity contributes to radiotherapy resistance and tumor growth.1(139) However, beta-hydroxybutyrate directly obstructs the synthesis of NLRP3, which reduces its inflammatory cytokine production and overall inflammatory response.1(139)
In conclusion, cancer is a leading cause of disease and mortality accounting for millions of deaths, worldwide. Gliomas, an often-malignant type of brain cancer, are a frequently diagnosed form of tumor. Although conventional interventions (chemotherapy, radiation therapy) show varying levels of success, the implementation for KDs as an adjunct modality, might be indicated for gliomas based on the unique biochemical and physiological processes therein. Broad-spectrum approaches targeting multiple signaling pathways unique to tumor cells (i.e., increased levels of glucose and insulin, increased oxidative stress, increased inflammation) might be indicated. Ultimately, KDs could serve to facilitate control of such degenerative cellular physiology.
References
1. Poff A, Koutnik AP, Egan KM, et al. Targeting the Warburg effect for cancer treatment: Ketogenic diets for management of glioma. Seminars in Cancer Biology. 2019;56:135-148. doi: https://doi.org/10.1016/j.semcancer.2017.12.011.
-Michael McIsaac
