
Modern Western diets, including macronutrient ratios and macronutrient quality, are substantially different than the hominin diets 10,000 years ago (Ilich, Kelly, Kim, & Spicer, 2014). Modern diets tend to include over 70% of total energy from refined sugars, refined vegetable oils, processed foods, and alcohol (Ilich et al., 2014). Of particular interest is the shift in polyunsaturated fatty acid (PUFA) ratios circa early to mid nineteenth century, after the inclusion of grain in animal diets and the development of processed foods.
Such a change increased the prevalence of omega-6 fatty acids in both meat and packaged food products (Ilich et al., 2014). Since then, PUFA ratios between omega-6 and omega-3 have shifted from 1-2:1 to as high as 15:1 in modern diets, respectively (Ilich et al., 2014). The aforementioned change in PUFA ratios has been linked to several chronic diseases, such as obesity. In an effort to gain a deeper appreciation of the deleterious effects of the modern diet, I would like to explore characteristics of omega-6 fatty acids, overconsumption, obesity, and their contributions to a phenomenon known as low-grade chronic inflammation (LGCI).
As noted in the introduction, unnaturally high levels of omega-6 (i.e., linoleic acid) PUFA consumption can develop obesity, as well as other conditions (Ilich et al., 2014). However, what are the mechanisms behind such a process? Ilich et al. (2014) cited that omega-6 to omega-3 ratios as high as 15:1, respectively, can initiate a process known as inflammation.
Acutely, inflammation is a natural immune response to neutralize and clear foreign bodies and damaged cells in the body (Ilich et al., 2014). However, prolonged states of inflammation, as seen by the frequent overconsumption of omega-6 PUFAs, can have systemic and deleterious effects on the body.
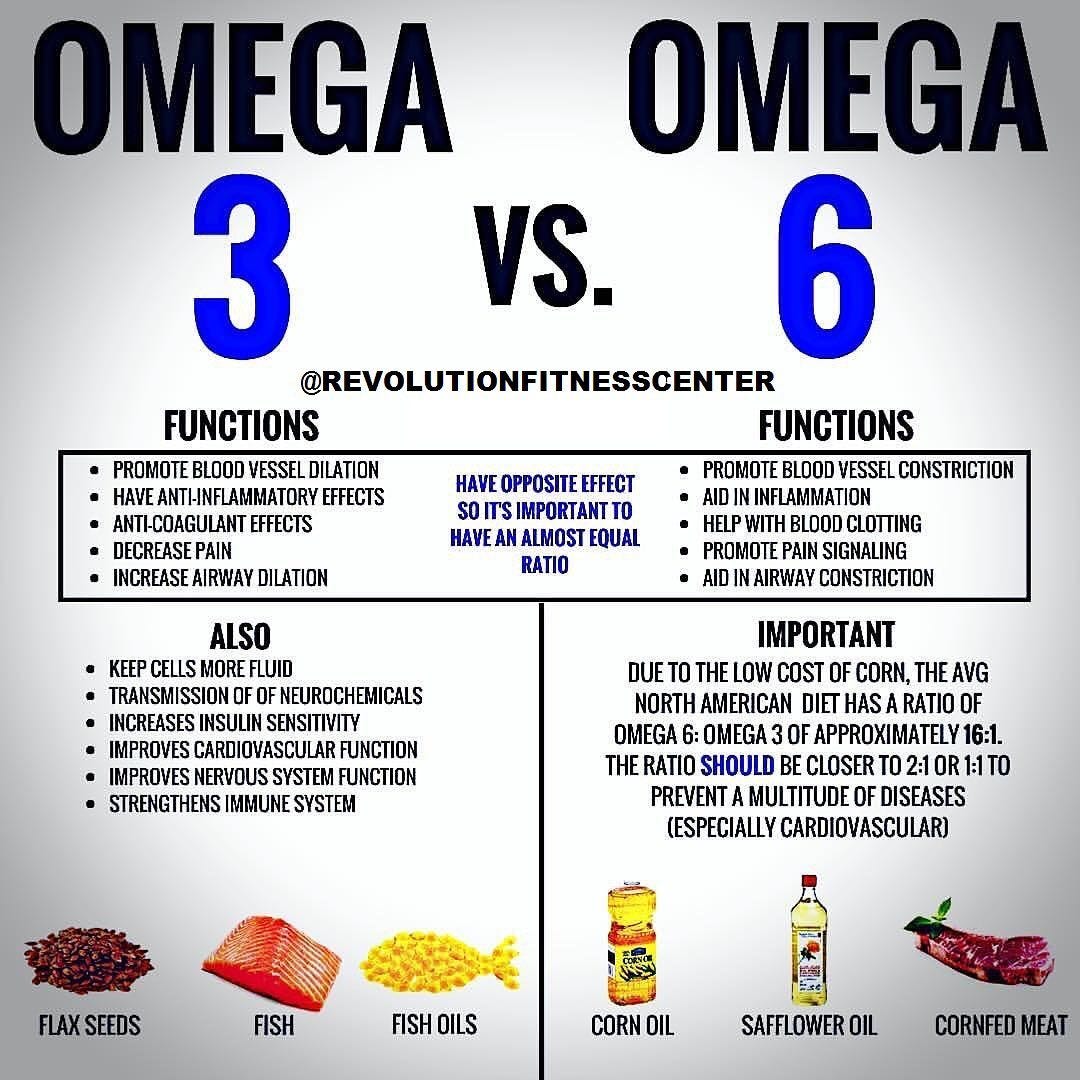
Briefly, omega-6 and omega-3 PUFAs undergo a biochemical change once absorbed. The metabolic products of both PUFAs include eicosanoids; signaling molecules responsible for the control of immune and inflammatory reactions in the body. Pro-inflammatory Omega-6 eicosanoids include prostaglandin E2, leukotriene B4, and thromboxane A2. Eicosanoids are generated by the enzymes lipoxygenase-5 and cyclooxygenase-2 (Ilich et al., 2014). Omega-3 eicosanoids, which are anti-inflammatory in nature, include prostaglandin E3, leukotriene B5, resolvins and protectins.
Here, anti-inflammatory eicosanoids also use lipoxygenase-5 and cyclooxygenase-2 (Ilich et al., 2014). Of particular relevance and interest are the shared enzymes between the two PUFAs (i.e., lipoxygenase-5 and cyclooxygenase-2); both PUFAs compete for use of lipoxygenase-5 and cyclooxygenase-2. Thus, if more omega-6 fatty acids are present in the diet than omega-3 fatty acids, more pro-inflammatory signaling molecules are created, and larger levels of systemic inflammation will occur.
If chronic consumption of high omega-6 / omega-3 ratios continue, chronic systemic inflammation ensues (Ilich et al., 2014). An inflammatory response also produces cytokines such as tumor necrosis factor alpha, interleukin-6, and interferon-gamma; proteins important in cell signaling and immune responses (Ilich et al., 2014). Low-grade chronic inflammation (LGCI) from overconsumption of omega-6 PUFAs perpetuates the production and levels of these pro-inflammatory signalling molecules.
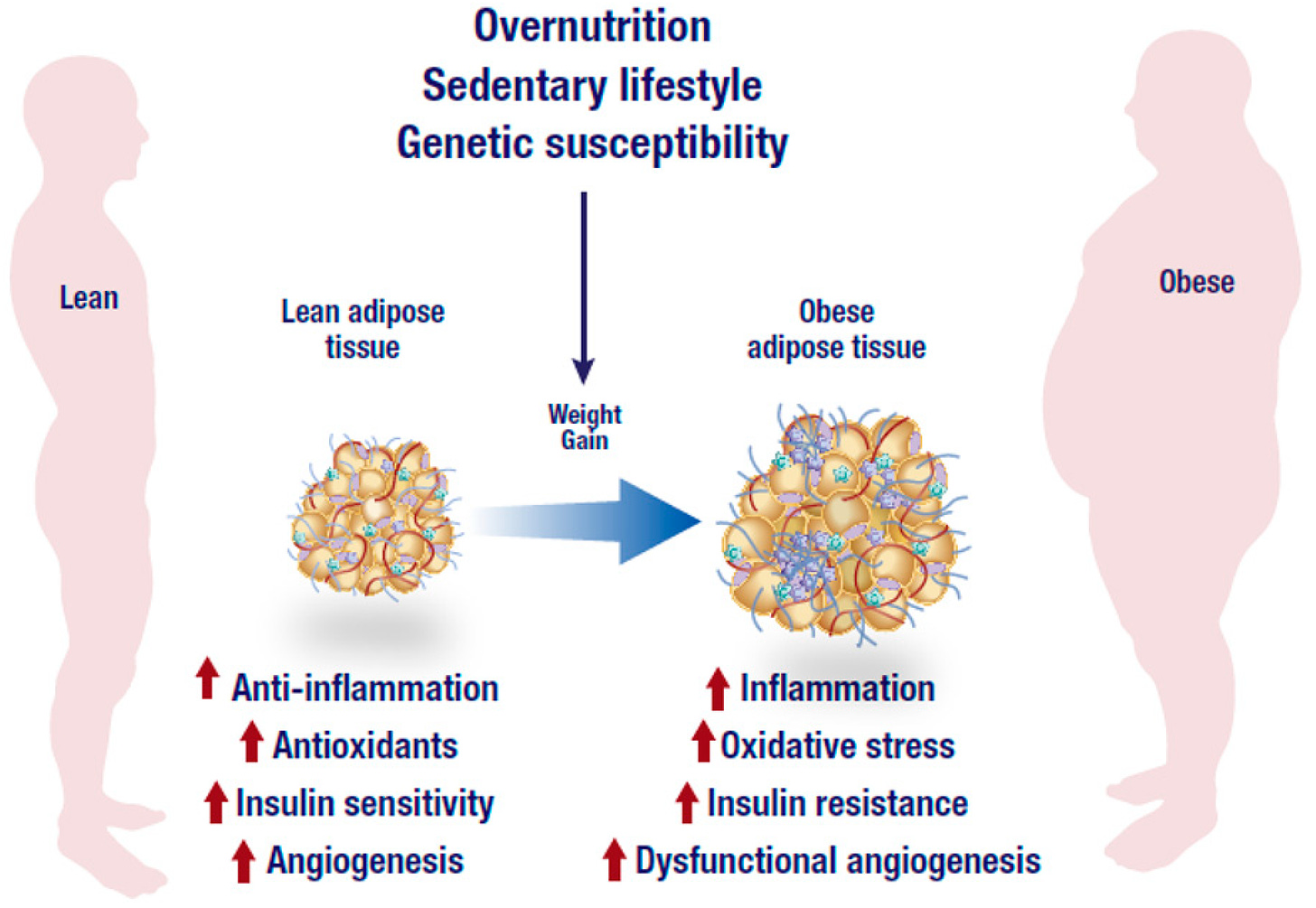
A relationship exists between LGCI and obesity; obese individuals have higher levels of inflammatory markers such as tumor necrosis factor alpha, and interleukin-6 as well as others such as C-reactive protein, adiponectin, and leptin (Ilich et al., 2014). Ilich et al. (2014) also noted that these overexpressed cytokines are produced in the adipose tissue of obese individuals; thus, more body fat produces more cytokines.
Additionally, there exists a constant interaction between adiponectin (anti-inflammatory) and leptin (pro-inflammatory) molecules. The coexistence of these cytokines perpetuates low-grade chronic inflammation. Thus, a feedback loop exists whereby obesity perpetuates inflammation, and inflammation perpetuates obesity (Ilich et al., 2014).
The article by Ilich et al. (2014) suggested that consistent overconsumption of omega-6 PUFAs has strong correlations to LGCI and obesity. If individuals who are overweight embark on a nutritional plan that facilitates an omega-6 to omega-3 ratio closer to the Paleolithic period (i.e., 1-2:1, respectively), such a rebalance of ratios may help reduce LGCI, body fat, and other conditions associated with LGCI.
References
Ilich, J.Z., Kelly, O.J., Kim, Y., & Spicer, M.T. (2014). Low-grade chronic inflammation perpetuated by modern diet as a promoter of obesity and osteoporosis. Archives of Industrial Hygiene and Toxicology, 65(2), 139-148.
-Michael McIsaac
