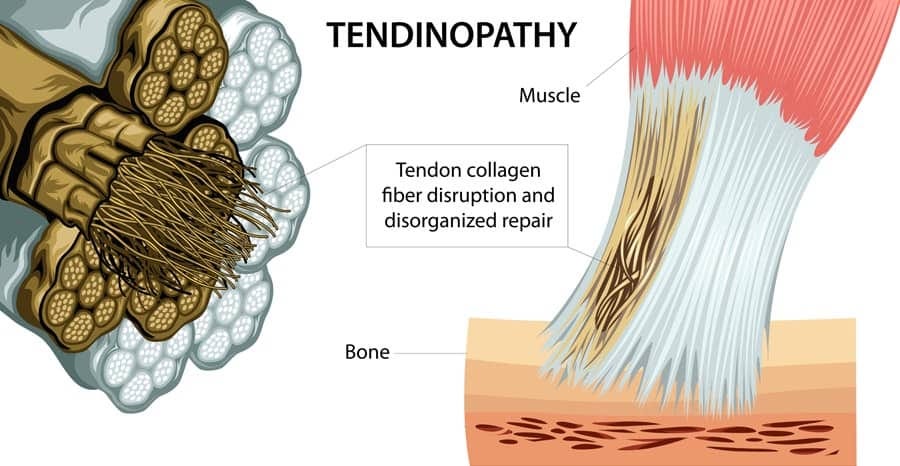
Tendinopathy is characterized as an overuse injury, occurring in close proximity to the tendinous regions of muscle bellies (Andres & Murrell, 2008). Unlike tendonitis, which is characterized by inflammatory markers, tendinopathy has minimal-to-no inflammation present (Andres & Murrell, 2008; Mayo Clinic, 2016). Thus, it is imperative to discern the two pathologies and appropriately characterize the condition, based on its complete presentation, not just pain. The following examination discusses how histological, biochemical, and biomechanical changes affect an individual experiencing pain and dysfunction affiliated with a tendinopathy, as well as look at causes and treatment options.

Histology can be defined as a branch of anatomy, which seeks to understand the structure and organization of tissues on a microscopic level (Histology, 2016). Andres and Murrell (2008) stated that tendinopathy, from a histological perspective, could be characterized by a degeneration and disorganization of collagen fibers, increased cellularity, in addition to minimal inflammation (a characteristic not found in tendonitis).
Macroscopically, tendinopathy is characterized by tendon thickening, in addition to a loss of mechanical properties (i.e., derived from stress and strain testing using rat models) and the presence of pain (Andres & Murrell, 2008; Reuther et al., 2015). In addition to a brief overview of histological changes characteristic of tendinopathy, the following section will also consider biochemical changes, and its implications.
Biochemically, Andres and Murrell (2008) noted unique changes, including the production of matrix metalloproteinases (MMPs) (substances involved in the degradation and breakdown of the extracellular matrix), tendon cell apoptosis (cell death), chondroid metaplasia (a benign change that occurs as a response to chronic physical or chemical irritation) in addition to protective factors such as insulin-like growth factor and nitric oxide synthetase (Andres & Burrell, 2008; Craig, Zhang, Hagood, & Owen, 2015; Farias et al., 2010).
It is also relevant to note note the co-existence of regenerative responses verses the pathologic responses from the body’s immune system; there is a net result in tendon degeneration. Ultimately, and most importantly, such changes lead to weakness, tearing, and pain (Andres & Murrell, 2008). Having considered the histological and biochemical characteristics of tendinopathy, the following section will review the third method of analysis (i.e., biomechanical) through stress-strain testing.
Furthermore, stress-strain testing will be used not only as a means of understanding the characteristics and behaviors of tissue when it fails; it will also be considered as a cause of tendinopathy in uncontrolled environments, known as load-induced tendinopathy (LIT).

Biomechanical analysis of tissues, via stress-strain testing, is a third method of understanding the characteristics of a tissue. Stress can be defined as a measure of the force (pounds per square inch) applied over a given cross-sectional area of a material, or specimen (Oatis, 2016). Such calculations are paramount, because they allow researchers to discover how much stress that tissues, like tendons, can withstand just before their behaviors begin to change (i.e., fail).
Such behaviors can be understood through ultimate strength (force required for a specimen to “break”), as well as elastic and plastic deformities, which will be covered in the following sections (Oatis, 2016). Goom, Malliaras, Reiman, and Purdam (2016) noted the critical importance of doing a sport-specific movement, walking, or running analysis as some movements can lead to excessive load on a tendon experiencing a tendinopathy.
Strain can be understood as a change in the original length of a material. Specifically, strain is defined as a change in length of a material divided by the original length, and is often presented as a percent change (Oatis, 2016). Strain, like stress, also provides valuable insight into other behaviors of tissues such as elastic and plastic deformation. Elastic deformation can be defined as the ability of a specimen to return to its original length after loading has been returned to zero.
Plastic deformation is defined as a specimen reaching a new length after loading has ceased while maintaining the new length (Oatis, 2016). In essence, stress and strain have a symbiotic relationship (i.e., stress can produce strain, and a change in strain may change the degree of stress a material can withstand). Such knowledge prepares one to better understand and appreciate the presentation of LIT, whose genesis can be derived from stresses and strains, in addition to histological and biochemical alterations.
LIT, as indicated, occurs from stresses placed upon the tendon (Cook & Purdam, 2009). Interestingly, LIT does not have a truly defined set of loads that induce the pathology; Cook and Purdam (2009) noted LIT can come from varying degrees of loads. Moreover, LIT can cause various levels of pain, irritability, and functional limitations, making it difficult to generalize and categorize. Tendon injury typically occurs within the mid-tendon, whereas other similar pathologies occur in locations more proximal to the tendinous point of insertion (Cook & Purdam, 2009). Loading of tendons has been shown to cause anabolic and catabolic reactions on the same.

Proximal hamstring tendinopathy (PHT) is a form of load-induced tendinopathy indicated by Goom et al. (2016). Goom et al. (2016) discovered that the etiology of tendinopathy could be attributed to extrinsic factors such as increasing volume and/or intensity too rapidly or a sudden introduction of sprinting, lunging, hurdles, or hills, in addition to intrinsic factors.
Such factors, as Goom et al. (2016) noted, precede the aforementioned training errors, involving the hamstring contracting and/or lengthening while in hip flexion, which can lead to provocative tensile and compressive loading at the tendon insertion (i.e., excessive static stretching involving end-range hip flexion or compressive loading from sitting). The findings of Goom et al. (2016) supported Cook and Purdam’s (2009) study (described below) in that storage of elastic energy, if improved, can reduce tendinopathies. Such findings will be discussed in the following sections.
Cook and Purdam (2009) indicated that energy storage, release, and excessive compression appear to be related to the onset of LIT, with particular emphasis on the volume (hours) and frequency (sessions per day/week) of loading. Moreover, the pathology is likely to be modulated by other factors such as genetics, age, hormones, sex, and body composition (Cook & Purdam, 2009). Thus, LIT is a complex condition, and is unlikely to have a singular, unified, solution or treatment. Having considered the biochemical, biomechanical, histological characteristics, and presentations of LIT, the following will consider treatment options for the aforementioned pathology.
Due to the multiple variations associated with the presentation of tendinopathy, no singular treatment has been found to be effective in its entirety. Having considered the evidence, it is the authors’ opinion that treatment is often dependent on severity of symptoms, as well the degree of despondency the individual is experiencing. Treatment options include: non-steroid anti-inflammatory drugs (NSAIDs), corticosteroid injections, diathermy, and physical therapy involving eccentric strengthening. The following treatments include medication or injections that are pain relieving, but have not been consistent in rehabilitation of a tendon.
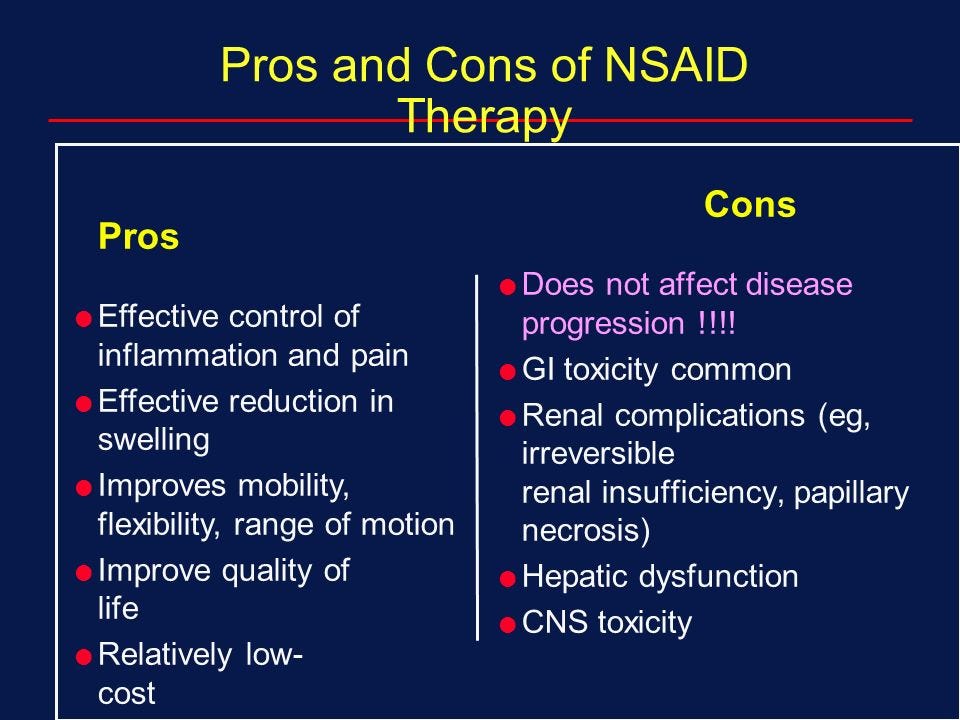
A prevalent first-line defense includes the use of NSAIDs; while effective in reducing pain temporarily they have shown inconclusive results when considering the underlying causes of tendinopathy and long term relief (Andres & Murrell, 2008; Boudreault et al., 2014). As tendinopathy presents itself with little-to-no inflammation, NSAIDs may not be addressing the entirety of the injury. Furthermore, due to the potential gastrointestinal and cardiovascular side-effects caused by NSAIDS, long term ingestion is ill advised (Andres & Murrell, 2008; Boudreault et al., 2014).
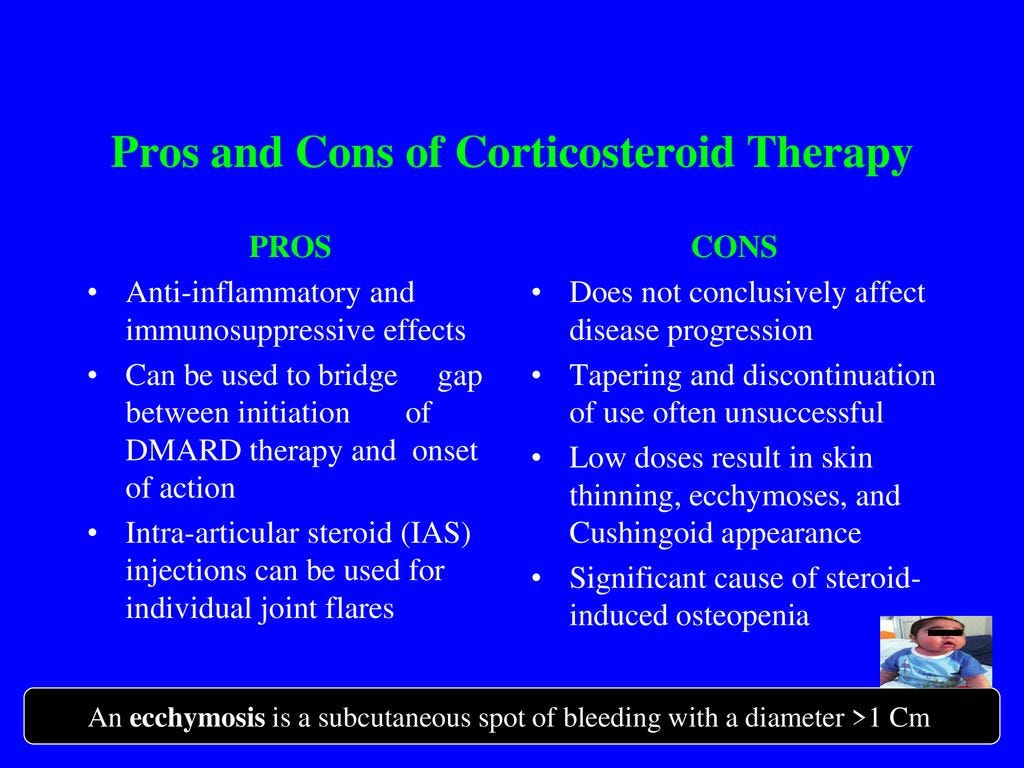
Corticosteroid injections have been widely used in the treatment of tendinopathy, despite ongoing concerns as to its safety and effectiveness (Andres & Murrell, 2008). Such an intervention could exist in the same category/effectiveness as NSAIDs, as temporary relief from pain is obtainable, although long-term repair of tendon tissue has not been proven (Boudreault et al., 2014). Therefore, corticosteroids may not result in long-term relief, but are useful for short-term pain alleviation (Andres & Murrell, 2008). Having considered pharmacological treatments and their advantages and limitations, the following will consider non-invasive treatments in addition to their strengths and weaknesses.

Diathermy is a non-invasive treatment, which involves heating deep tissue while cooling superficial tissue simultaneously. The heat stimulates blood flow, which promotes healing while the cooling keeps the tissue from overheating (Szlosek, Taggart, Cavallario, & Hoch, 2014). Szlosek et al. (2014) found that diathermy was equally effective as corticosteroids, thereby providing temporary relief of pain, but is inconclusive in its efficacy to heal tendon. Szlosek et al. (2014) also suggested that diathermy, as a stand-alone modality may not be sufficient in alleviating tendinopathy; additional therapies, such as stretching and range-of-motion exercises may be advised.

Physical therapy has been implemented as treatment option for many tendinopathies. A relatively recent trend in the aforementioned intervention included eccentric strengthening (Andres & Murrell, 2008). Physical therapies, involving strengthening exercises, have been used to treat tendinopathy, but with limited success (Andres & Murrell, 2008).
Specifically, eccentric exercise applied by having clients slowly lengthen the affected muscle(s) out of a contracted state has been a preferential choice of physical therapists (Andres & Murrell, 2008). Ortega-Castillo and Medina-Porqueres (2016) found studies, which examined eccentric exercise and tendinopathy failed to specify parameters, such as sets, repetitions, and rest periods, associated with the treatment. Furthermore, locating randomized control trials, which did not combine eccentric exercise with other modalities was also sparse, thus questioning its effectiveness (Ortega-Castillo & Medina-Porqueres, 2016).
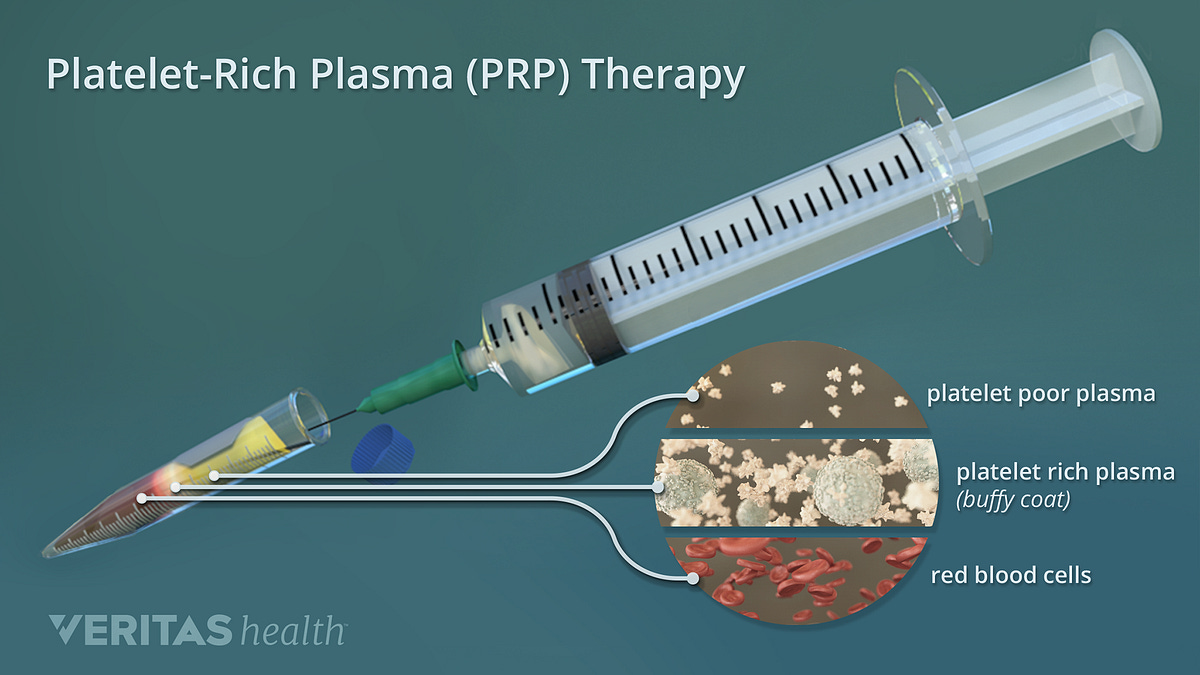
A relatively new second-line, and more invasive treatment for tendinopathy is platelet-rich plasma (PRP) injections (Andia & Maffulli, 2016). The aforementioned injections are thought to play a role in prohibiting the progression of tendinopathy by introducing cells, which may aid in healing tendons (Andia & Maffulli, 2016; Nourissat et al., 2015). Implemented primarily in more persistent cases, PRP shows a degree of promise, though research has not provided absolute conclusive evidence regarding the same (Andia & Maffulli, 2016; Nourissat et al., 2015).
Andia and Maffulli (2016) noted that the heterogeneity of tendinopathy and the various tissues that can be affected, PRP still needs refinement to advance it as a viable treatment. Having considered the pharmacological and non-invasive treatments thus far in the preceding sections, the following will consider surgery as a final intervention in addition to its advantages, disadvantages, and limitations.
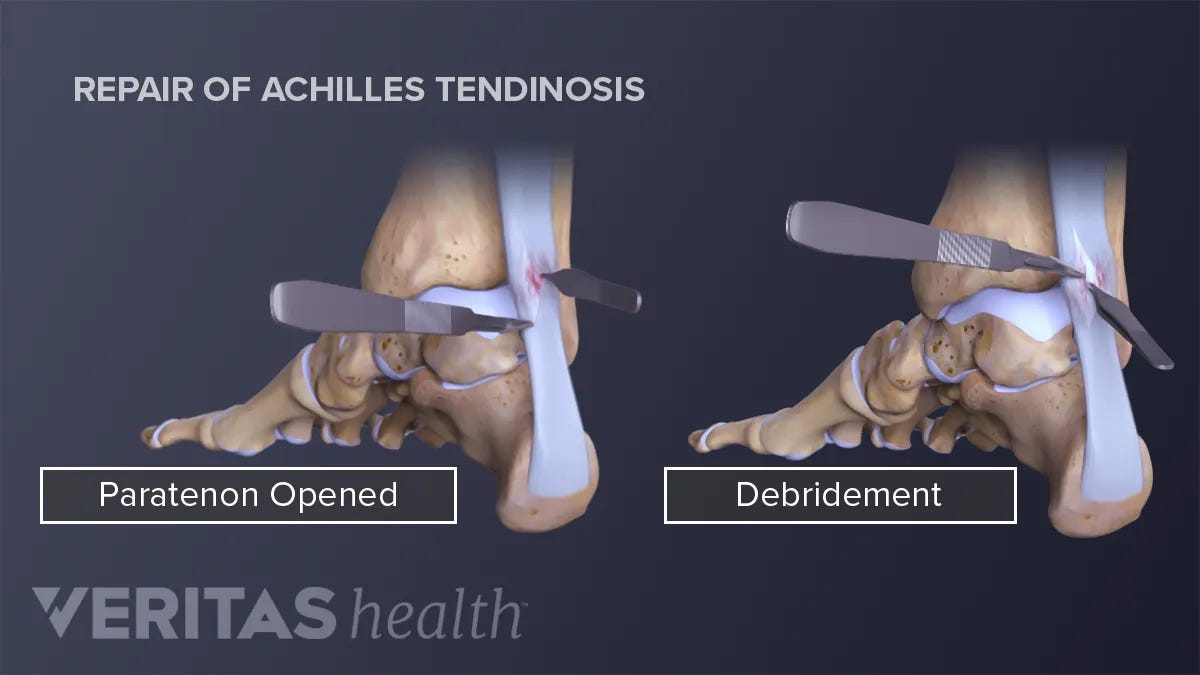
Considered a last resort, due to its high cost and risk, surgical treatment for tendinopathies are inconclusive and varied (Andres & Murrell, 2008). A commonly used surgical procedure, called debridement, involves removal of damaged tissue in order to improve healing in the remaining healthy tissue (Andres & Murrell, 2008). The aforementioned surgery can be performed arthroscopically or in a more traditional open tissue fashion (Andres & Murrell, 2008). While improvement has been shown in some instances, more randomized controlled studies are indicated to determine its effectiveness (Andres & Murrell, 2008). Additionally, such interventions have been efficacious only in cases where first and second lines of defense were proven unsuccessful (Andres & Murrell, 2008).
In essence, absolute, definite, and ubiquitous conclusions regarding tendinopathy treatments remain elusive. Thus, treatment is often dependent on severity of symptoms, as well the degree of despondency the individual is experiencing. Despite the complexity of tendinopathy, there does exist a progression of interventions. First line defense strategies such as NSAIDs, diathermy, corticosteroid injections, and physical therapy may help with pain and provide short-term, and in some cases, long-term relief.
Other individuals with more persistent and serious symptoms may consider PRP or injections. Finally, surgery may act as an option for those with more obstinate cases of tendinopathy (Andres & Murrell, 2008). Despite the aforementioned interventions, evidence-based research and best practice suggests that individuals exhaust less aggressive treatments first, as they have they contain within them, the smallest of side effects and risk. Ultimately, such pragmatic steps would allow the possibility of liberating individuals from pain, while minimizing complications, and enhancing quality of life.
References
Andia, I., & Maffulli, N. (2016). Clinical outcomes of biologic treatment for chronic tendinopathy. Operative Techniques in Orthopedics, 26(Small Molecule Biologic and Gene Based Treatments in Sports Medicine), 98-109. http://dx.doi.org/10.1053/j.oto.2015.12.007
Andres, B.M., & Murrell, G.A.C. (2008). Treatment of tendinopathy: What works, what does not, and what is on the horizon. Clinical Orthopedics and Related Research, 466,1539-1554. http://dx.doi.org/10.1007/s11999-008-0260-1
Boudreault, J., Desmeules, F., Roy, J., Dionne, C., Frémont, P., & Macdermid, J. C. (2014). The efficacy of oral non-steroidal anti-inflammatory drugs for rotator cuff tendinopathy: a systematic review and meta-analysis. Journal of Rehabilitation Medicine, 46(4), 294-306. http://dx.doi.org/10.2340/16501977-1800
Cook, J.L., & Purdam, C.R. (2009). Is tendon pathology a continuum? A pathology model to explain the clinical presentation of load-induced tendinopathy. British Journal of Sports Medicine, 43(6), 409-416. http://dx.doi.org/10.1136/bjsm.2008.051193
Craig, V.J., Zhang, L., Hagood, J.S., & Owen, C.A. (2015). Matrix metalloproteinases as therapeutic targets for idiopathic pulmonary fibrosis. American Journal of Respiratory Cell and Molecular Biology, 53(5), 585-600. http://dx.doi.org/10.1165/rcmb.2015-0020TR
Farias, L.C., Bonan, P.R.F., Martelli, H., Santos, L.A.N., De-Paula, M.B., & Guimaraes, A.L.S. (2010). Unusual nodular lesion of the tongue: clinical report of chondroid metaplasia. Revista de Clínica e Pesquisa Odontológica, 6(2), 175-178. Retrieved from https://scholar.google.ca/scholar?cluster=6529421117652471709&hl=en&as_sdt=0,5&sciodt=0,5
Goom, T., Malliaras, P., Reiman, M., Purdam, C. (2016). Proximal hamstring tendinopathy: Clinical aspects of assessment and management. Journal of Orthopaedic & Sports Physical Therapy, 46(6), 483-493. http://dx.doi.org/10.2519/jospt.2016.5986
Histology (2016). In Merriam-Webster. Retrieved from http://www.merriam-webster.com/dictionary/histology
Mayo Clinic (2016). Diseases and conditions: Tendonitis. Retrieved from http://www.mayoclinic.org/diseases-conditions/tendinitis/basics/definition/con-20020309
Nourissat, G., Ornetti, P., Berenbaum, F., Sellam, J., Richette, P., & Chevalier, X. (2015). Review: Does platelet-rich plasma deserve a role in the treatment of tendinopathy? Joint Bone Spine, 8, 2230-234. http://dx.doi.org/10.1016/j.jbspin.2015.02.004
Oatis, C.A. (2016). The mechanics and pathomechanics of human movement (3rd ed.). New York, NY: Lippincott Williams & Wilkins.
Ortega-Castillo, M., & Medina-Porqueres, I. (2016). Effectiveness of the eccentric exercise therapy in physically active adults with symptomatic shoulder impingement or lateral epicondylar tendinopathy: A systematic review. Journal of Science & Medicine in Sport, 19(6), 438-453. http://dx.doi.org/10.1016/j.jsams.2015.06.007
Reuther, K.E., Thomas, S.J., Tucker, J.J., Vafa, R.P., Gordon, J.A., Liu, S.S,.… Soslowsky, L.J. (2015). Overuse activity in the presence of scapular dyskinesis leads to shoulder tendon damage in a rat model. Annals of Biomedical Engineering, 43(4), 917-928. http://dx.doi.org/10.1007/s10439-014-1137-y
Szlosek, P. A., Taggart, J., Cavallario, J. M., & Hoch, J. M. (2014). Effectiveness of diathermy in comparison with ultrasound or corticosteroids in patients with tendinopathy: A critically appraised topic. Journal of Sport Rehabilitation, 23(4), 370-375. http://dx.doi.org/10.1123/JSR.2013-0063
-Michael McIsaac
