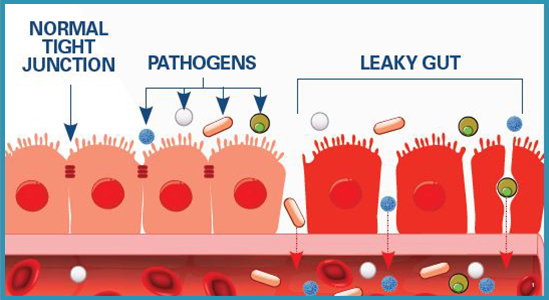
The intestinal wall, as has been discussed in previous posts, is composed of intestinal epithelium forming a semipermeable barrier preventing entry of pathogens and blocking contact with constituents of the immune system.1 Ultimately, such a system facilitates normal digestion/absorption, immune function, and overall homeostasis. However, disruption to the intestinal barrier can induce unfavorable changes to gut health. As a means of appreciating the epithelial barrier, the following will consider how antibiotic use, a necessary medication for certain conditions, can have side-affects to include disrupted gut function (with focus on leaky gut) and downstream pathophysiological aberrations.
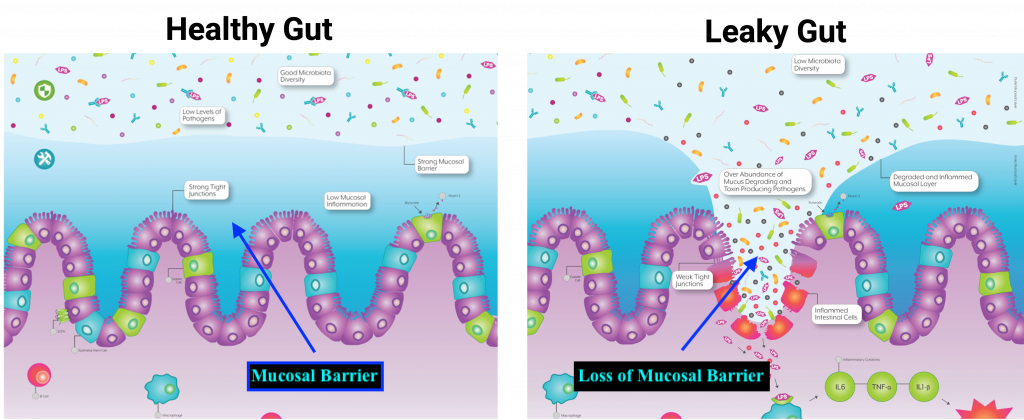
The lining of the intestinal tract not only block pathogens from undesired entry and contact with the immune system; such a mucosal barrier must also manage chemical and physiological impositions from gastric juices and pancreatic enzymes.2 As such, the epithelial cells of the outer cellular barrier are constantly replaced (among the highest cell turnover in the body) due to degradation, and must form tight junctions to maintain integrity.2(425) It is the tight junction structure that, when dysregulated, can allow pathogens and food particles to become undesirably close to components of the immune system.2(425)
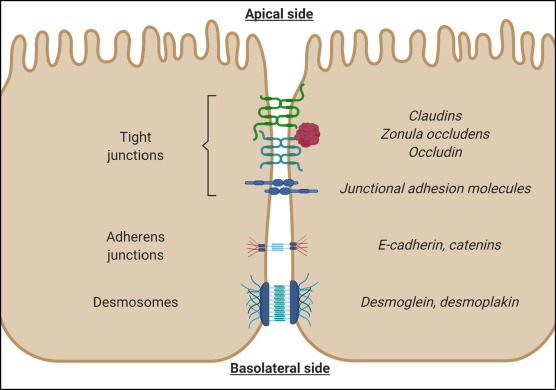
Tight junctions are composed of proteins known as zonula, occludens, and claudins; important components that help produce and maintain tight junction barriers.1(2) Loss of tight junction integrity is compromised and associated with several diseases to include severe burns, shock, intestinal injury, and inflammatory bowel disease (IBD).1(2) As discussed in this author’s previous posts, the microbiota is a term to describe an aggregate of viruses, bacteria, fungi, and archaea (single celled organisms similar to bacteria), which contribute to intestinal barrier function, nutrient absorption, restriction of pathogen colonization, and immune function.1(2) Thus, the microbiota plays a central role in intestinal homeostasis.

The microbiota can come under duress and altered function from dietary changes, infection, intestinal ischemia (restriction of blood supply), diseases, stress, and antibiotic use.1(2)Although antibiotic use has a time and place to treat bacterial infections, Feng et al.1(2) indicated that there can be serious consequences such as multi-drug resistant pathogens. However, secondary affects also included destruction of the microbiota with long-lasting effects not easily resolved.1(2) The research of Feng et al.1(2), using rat models, indicated that antibiotics also impaired intestinal microbiota homeostasis, activated NLRP3 (expressed in macrophages and detects damages cells) inflammasome, decreased short chain fatty acid content (energy source for the colon) contents, autophagy, and disrupted intestinal tight junctions.1(2)
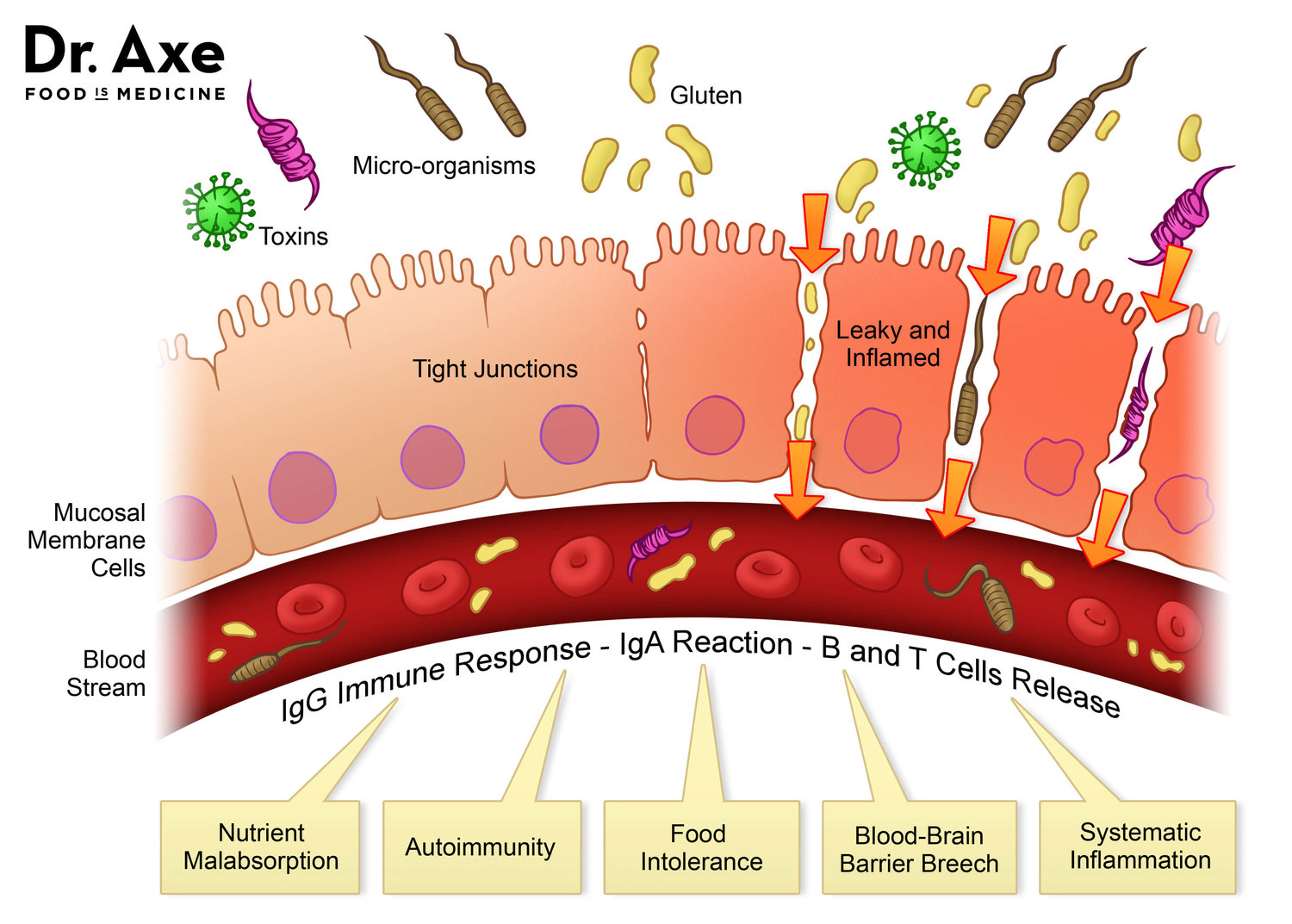
If tight junctions become less stable from antibiotic use, only 2% entry of luminal contents (i.e., undigested molecules such as proteins) past the sub-mucosal barrier between the can initiate an immune response.2(425) Macromolecules that passively transit between disrupted tight junctions must be degraded by Kupffer cells. Xenobiotics and microbial toxins that also can enter the bloodstream must move through phase 1 and phase 2 detoxification of the liver and eventual excretion via bile or urine.2(425) Ultimately, such excessive and usually unnecessary immune responses and detoxification processes place higher-than-normal demands on the body’s resources which requires antioxidants and glutathione. Prolonged and chronic processes as mentioned above can also produce reactive oxygen species (product of liver detoxification) which must be managed.2(426)
In conclusion, optimal intestinal tract function maintains normal digestion/absorption, immune strength, and homeostasis. However, disruption to the intestinal barrier tight junctions from antibiotic use can induce serious and unfavorable changes to the intestinal lining and whole-body health. Ultimately, such aberrations in tight junction integrity should highlight caution and vigilance when deciding to use such medications, urging the individual to weight the risks and benefits before use.
References
1. Feng Y, Huang Y, Wang Y, Wang P, Song H, Wan F. Antibiotics induced intestinal tight junction barrier dysfunction is associated with microbiota dysbiosis, activated NLRP3 inflammasome and autophagy. PLoS One. 2019:14(6);1-19. doi:10.1371/journal.pone.0218384.
2. Lord RS, Bralley, JA. Laboratory Evaluations for Integrative and Functional Medicine (2nded.). Duluth, GA: Genova Diagnostics; 2012.
-Michael McIsaac
