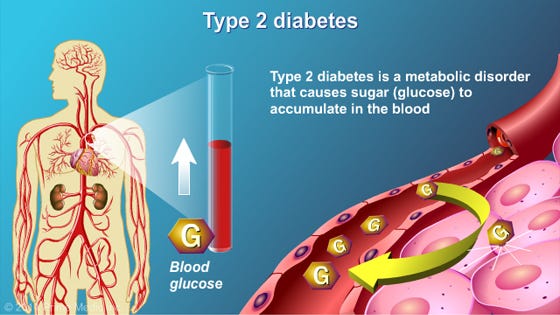
Technological progress is nested deeply within contemporary Western society, and has brought with it many conveniences gifted to its citizens; acute-care medicine, the combustion engine, mass food production, enhanced communications via smartphones, and instant access to information through the Internet, to name a few. Although such examples show technological prowess, they still remain but a modest sampling of total innovations found within the fabric of 21rst century civilization. Despite such growth in the post-industrial era, Western society has not been without its challenges. Ironically, there exists an incongruence between innovation and chronic disease; modern conveniences have likely given birth to aberrations in human health and longevity, such as type 2 diabetes (T2D). As a means of understanding and contextualizing T2D as a chronic disease of civilization, this author would like to present its underlying etiology, risk factors, and methods of assessment. Most importantly, solutions will be presented to help facilitate recovery and liberation from T2D.
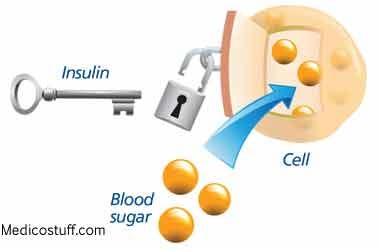
T2D is characterized by the body’s decreased ability to store glucose in the presence of insulin, also known as insulin resistance (Reisner & Reisner, 2017). Over time, insulin output from the beta cells of the pancreas (produce and secrete insulin) increases (hyperinsulinemia) to compensate for the target tissues’ lack of sensitivity to said hormone (D’Adamo & Caprio, 2011). As insulin sensitivity continues to deteriorate, the output of insulin from the beta cells becomes unable to meet the demand of target tissues (Reisner & Reisner, 2017; Geragotou, et al., 2016). Eventually, beta cells become dysfunctional and un-stored glucose concentrations begin to rise in the bloodstream, inducing a phenomenon known as hyperglycemia (Reisner & Reisner, 2017). Such conditions define T2D and provide a fertile ground for diabetic complications to the vasculature, nervous system, kidneys and retina (Russell & Cooper, 2015). Having considered the manifestations and ramifications of T2D, it is imperative that screening methods are implemented in those individuals thought to be at risk of developing such a disease. Thus, the following section will consider two diagnosis techniques, hemoglobin A1C (HbA1c) and the oral glucose test, considered gold standards when screening for pre-diabetes and diabetes (Hong, Ku, & Shong, 2015).
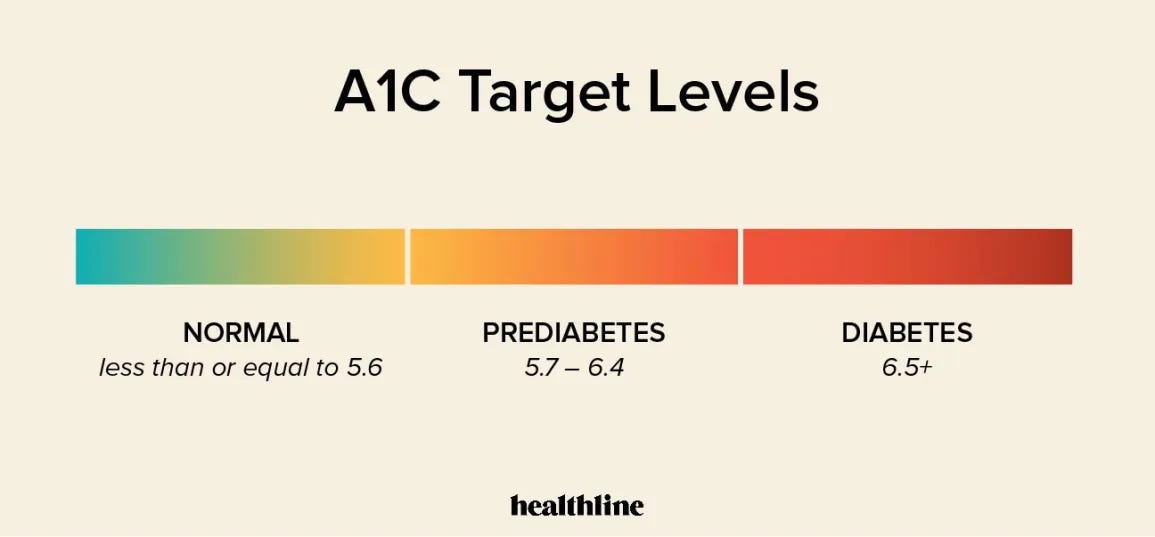
HbA1c is the preferred method of quantifying and diagnosing T2D (Canadian Task Force on Preventative Health Care, 2012). HbA1c measures blood glucose concentrations in the blood over a three-month period. When excessive blood glucose concentrations rise above normal levels (i.e., hyperglycaemia), it tends to bind to circulating red blood cells, a process known as glycation (Hemoglobin A1c (HbA1c) Test for Diabetes, 2016). Such a measure allows for a determination of the risk and severity of T2D, and a HbA1c of 6.5% or greater is considered the threshold for diagnosing diabetes (Canadian Task Force on Preventative Health Care, 2012).
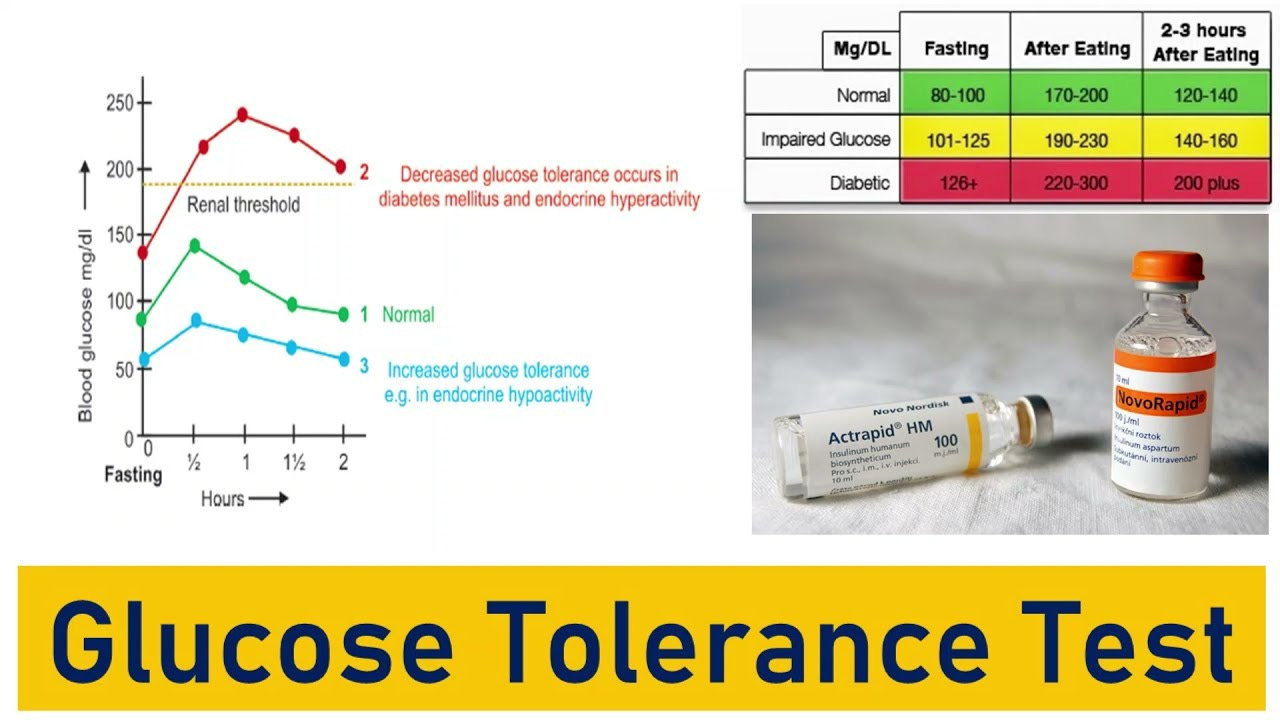
The oral glucose test, considered a second gold standard, is another means of helping determine the risk and/or degree of T2D (Hong, Ku, & Shong, 2015). The glucose test is administered by providing the patient with 75grams of glucose in form of a solution, which is consumed after an initial blood glucose test is performed (to compare pre and post glucose concentrations) (Iwao, Sakai, & Ando, 2013). Iwao et al. (2013) indicated that two hours after the glucose solution is ingested, a blood sample is gathered. If plasma glucose ranges from 140-199mg/dL, such a concentration is considered impaired glucose tolerance. If plasma glucose ranges from 200mg/dL or higher, it is considered diabetes (Iwao et al., 2013). Having considered the pathophysiology and screening methods of T2D, the following will explore the epidemiology of such a disease.
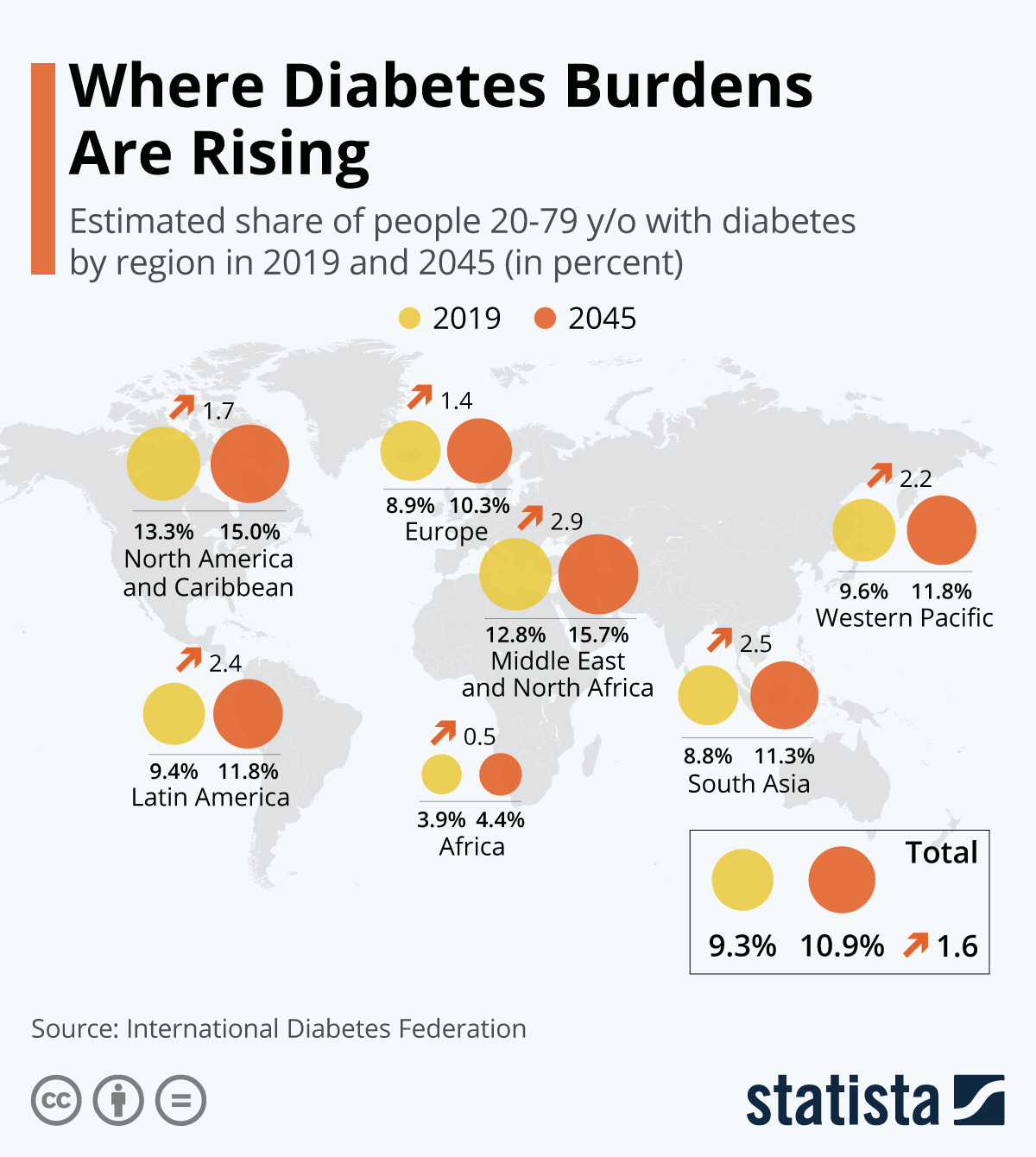
The underlying cause of T2D remains uncertain, and at best, speculative (Reisner & Reisner, 2017). However, epidemiological research has provided relevant insights of those afflicted with T2D (D’Adamo & Caprio, 2011). Though not entirely causative in nature, epidemiological studies have served as a foundation for deeper research, and as a point of reference to initiate interventions. What is known about T2D is that it affects approximately 382 million people worldwide, with another 175 million cases undiagnosed (Mattei et al., 2015). T2D also tends to occur in lower to middle income families, and increases in the disease are expected to rise in the next 20-40 years (Mattei et al., 2015).

Individuals with T2D tend to be obese, and increased adiposity is occurring in greater numbers, especially across paediatric populations (D’Adamo & Caprio, 2011). Insulin resistance (characteristic of T2D) is also associated with physical inactivity and is intimately connected with metabolic syndrome (MS); a cluster of health complications associated with T2D, heart disease and other heart-related problems (Amuta, Crosslin, Goodman, & Barry, 2016; Kenney, Wilmore, & Costill, 2012). Finally, T2D is strongly associated with poor nutritional choices and habits, especially increased fat and refined carbohydrate consumption (Mattei et al., 2015). When combined, obesity, inactivity, and overconsumption of processed carbohydrates provides a fertile ground for the genesis of T2D. The following will consider each constituent in greater detail and its context within developed countries.
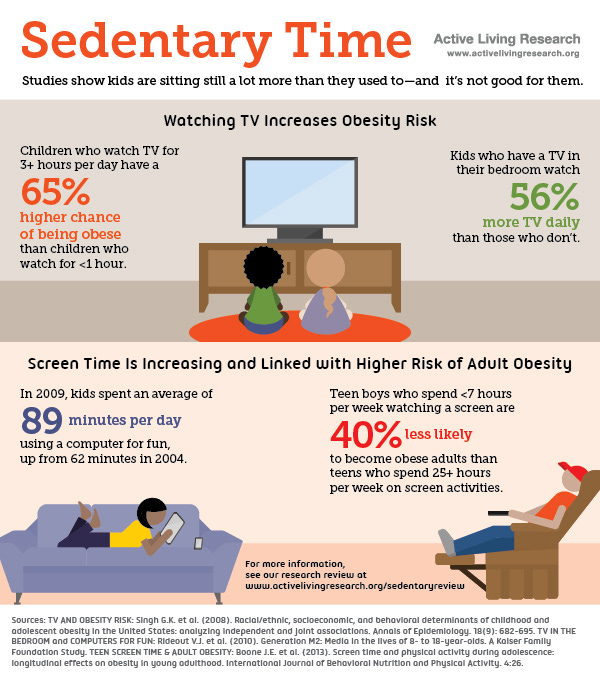
Inactivity is deeply imbedded within Western society. Such sedentary behavior finds its beginnings in preschool, into post-secondary education, and throughout much of the work force. One study of Chinese children in seven Chinese provinces uncovered that they were studying (seated positions) as long as ten hours per day, which included home-based study and school (Zhang, Seo, Kolbe, Middletadt, & Zhao, 2011). Furthermore, Matthews et al. (2008) discovered that from 6,329 participants in the study between the years 2003 and 2004, an average of 7.7 hours a day was spent between seated and sedentary activities. Another study with 222 children (12-16 years of age) indicated that watching television for more than 2 hours a day had a significant correlation to increased consumption of fast foods, sugar sweetened beverages, and a higher BMI (Alghadir, Gabr, & Iqbal, 2016). Finally, the Bureau of Labor Statistics (2013) stated that the average American spends at least 2.8 hours a day watching television after work. In essence, educational institutions, demands of seated work, and available time spent watching television are major contributors to increased processed food consumption and inactivity in Westernized civilizations.

Mass food production is another defining characteristic of first world countries; Western civilizations tend to derive approximately 70% of total daily energy from refined carbohydrate sources (Ilich, Kelly, Kim, & Spicer, 2014). Such sources include vegetable oils, sugar, alcohol, and processed foods, (Ilich et al., 2014). Interestingly, sugar, especially high fructose corn syrup (HFCS), has increased proportionate to obesity, MS, and diabetes (Ochoa, Lalles, Malbert, & Laillet, 2015). It is also hypothesized that hyperpalatibility of such foods containing refined sugars (and salt/fat) also interrupt natural signaling of satiety, typically found in natural whole foods (Ifland et al. 2009; Ochoa et al., 2015). Such a hypothesis provides a plausible explanation for overeating said foods. Interestingly, during the paleolithic era (~11,000 years ago), individuals consumed approximately 73% of their total daily calories from animal sources, largely devoid of carbohydrates (Cordain, Miller, Eaton, Mann, Holt, & Speth, 2000). Since then, there has been an inversion from which dominant macronutrient (and quality) calories are derived from (i.e., from protein to carbohydrates). Ultimately, it is possible that such abundance and access to refined foods in the modern era have contributed to an overabundance of calories in the human body, contributing to insulin resistance and obesity (both strongly associated with T2D).
Obesity, in and of itself, has strong associations with T2D, whether such a condition is induced by inactivity and/poor nutrition or via genetic drivers. Obesity can be defined as having excess body fat as a percentage of total weight (Kenney et al., 2012). Generally speaking, men are considered obese when they exceed 25% body fat, while women are considered obese when they exceed 35% body fat (Kenney et al., 2012). Since the 1970s, obesity has risen dramatically in the United States; 72% of males and 64% of females are obese (Kenney et al., 2012). Other countries are also developing into obese nations such as Canada, Europe, and Australia (all Westernized societies) (Kenney et al., 2012). Not only is such a condition widespread; it is also highly correlated to morbidity, mortality, insulin resistance, and T2D (Kahn, Hull, & Utzschneider, 2006; Meckling & Sherfey, 2007).
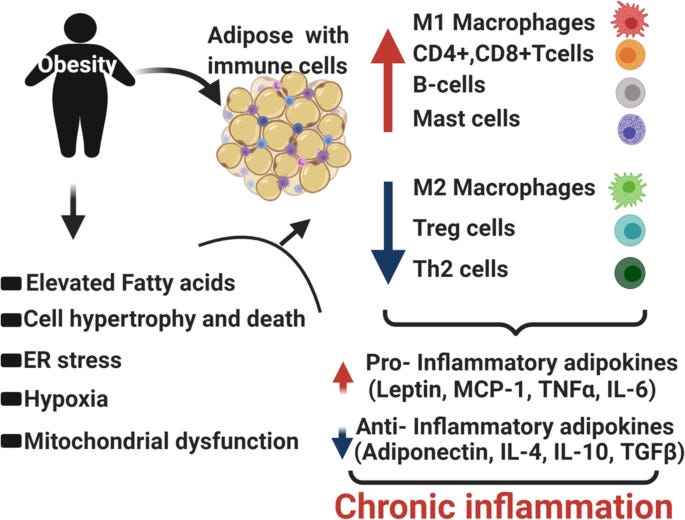
Individuals who are obese tend to release higher levels of substances from their adipose tissue that contributes to low-grade chronic inflammation (immune response) and insulin resistance (Kahn et al. 2006). Said substances include non-esterified fatty acids (NEFAs), glycerol, pro-inflammatory cytokines, and hormones (leptin and adiponectin). The release of NEFAs is likely to play a critical factor in controlling insulin sensitivity; increased NEFA levels are detected in obesity and type 2 diabetes, and are also associated with insulin resistance observed in both (Kahn et al. 2006). It is possible that NEFAs compete with serum glucose for use in cellular energy production, increasing blood glucose concentrations (Kahn et al. 2006). It has also been hypothesized that NEFAs interrupt insulin receptor signaling on the cell; increased fatty acid cellular metabolites (diacylglycerol, fatty acyl-coenzyme A, and ceramides) interact with key proteins (insulin receptor substrate-1, and insulin receptor substrate-2) that transmit signals from insulin to the inside of the cell (Kahn et al. 2006). In essence, it is thought that cellular metabolites diminish insulin-receptor cell signalling. Thus, obesity can likely drive insulin resistance. Having considered inactivity, inadequate nutrition, obesity, and their relationships to T2D, the following will consider solutions to mitigate and possibly reverse the chronic disease.
One method of controlling hyperglycaemia and obesity is through movement (Kenney et al., 2012). Physical activity also improves lipid profiles, cardiovascular markers, lean body mass, and endothelial function; other factors often associated with T2D (Lamb, 2014). Lamb (2014) stated that physical activity could help reduce the risk of developing T2D between 47-58% in high-risk groups, indicating the influence of exercise in controlling hyperglycemia. Interestingly, much of the research involving movement focused upon aerobic exercise and its influence upon insulin resistance (which is also highly effective). However, Lamb (2014) stated that resistance exercise also held relevance with regard to its influence upon T2D biomarkers; such a form of exercise (anaerobic energy production) has been shown to have acute affects upon insulin sensitivity (Lamb, 2014). A characteristic of T2D is a reduction of insulin receptors along cell membranes, also known as GLUT-4 glucose transporters, within target tissues (Kenney et al., 2012). Physical activity and muscle contraction produces insulin-like activity by increasing membrane permeability (Kenney et al., 2012). Such a process allows glucose to enter the membrane, evading the challenge of reduced GLUT-4 glucose transporters. General recommendations for physical activity, according to the American Diabetes Association Standards for Diabetes Care, includes at least 150 minutes of moderate-intensity exercise at approximately 50-70% maximum heart rate, 3 days per week (Lamb, 2014). Adults with T2D are also encouraged to engage in resistance training at least 2 days per week (Lamb, 2014). Having considered the influence of exercise and its influence upon T2D, the following will consider nutrition and its affects on biomarkers of the aforementioned disease.

As mentioned in previous sections, there has been a shift from natural/unprocessed foods towards overconsumption of processed items within Westernized countries (Ilich et al., 2014). There has also been a shift from drinking water in favor of sugar-sweetened beverages (Miller et al., 2013). Both alterations in food consumption and hydration have paralleled the rise of T2D in addition to obesity, systemic inflammation, cardiovascular disease, and MS (Miller et al., 2013). One particular method, which has shown promise, is implementation of a very low carbohydrate/ketogenic diet (VLCKD) (Paoli, Rubini, Volek, & Grimaldi, 2013). Individuals with T2D are characteristically resistant to the affects of insulin. VLCKDs, however, have been shown to circumvent the requirement of said hormone via ketosis while reducing fat mass (Paoli et al., 2013).
Unique to ketosis is the near absence/influence of insulin and glucose for energy production (Paoli et al., 2013). Ketosis is a process whereby the body predominantly adapts to using triglycerides, stored in adipose tissue, for adenosine triphosphate (ATP) production instead using glucose. Such an adaptation is achieved through a reduction of carbohydrates to less than 50 g/day (Paoli et al., 2013).
After approximately 3-4 days, the central nervous system and other tissues shift from using glucose as a primary substrate (because there is not enough circulating) to triglycerides from adipose tissue in the form of ketones: acetoacetate, acetone, and beta-hydroxybutyric acid (Paoli et al., 2013). A key feature of insulin resistance is an impaired ability of muscle cells to absorb circulating glucose. Additionally, individuals with insulin resistance will divert a greater proportion of dietary carbohydrates to the liver where is it converted to fat, thereby contributing to increased fat mass (Paoli et al., 2013).
Ketosis, the state of producing and using ketones as a main energy substrate, circumvents such glucose storage (as fat) since it does not require glucose or exogenous carbohydrate consumption for ATP production. Thus, VLCKDs help control deposition of fat, thereby controlling weight gain, in addition to improving insulin sensitivity (Paoli et al., 2013). It has also been postulated that higher fat and protein intake found in VLCKDs may have an appetite suppressing affect, further contributing to weight loss (Paoli et al., 2013). Physiologically, by reducing insulin secretion, lipogenesis (fat storage) is down regulated while lipolysis (release of triglycerides from adipose tissue) is up regulated; a possible mechanism of improving lean body mass and reducing hyperglycemia (Paoli et al., 2013).
Western culture has made exponential leaps in both medical and technological innovations within the last 150 years. However, such comforts and circumstances (i.e., mass processed food production, mechanized transportation, television, seated work) have likely contributed to overconsumption of food, inactivity, and obesity. Such a triad has been strongly associated to the genesis and manifestations of T2D. However, simple, cost-effective, and non-pharmaceutical interventions (i.e., VLCKDs, increased physical activity, fat loss) do exist in treating insulin resistance and T2D. Such knowledge should provide hope and incentive in liberating those from such a chronic disease.
References
A’Damo, E., & Caprio, S. (2011). Type 2 diabetes in youth: Epidemiology and pathophysiology. Diabetes Care, 34(2), 5161-5165.
Alghadir, A. H., Gabr, S. A., & Iqbal, Z. A. (2016). Television watching and body mass index of school children in Saudi Arabia. Pediatrics International, 58(4), 290-294.
Amuta, A. O., Crosslin, K., Goodman, J., & Barry, A. E. (2016). Impact of type 2 diabetes threat appraisal on physical activity and nutrition behaviours among overweight and obese college students. American Journal of Health Behaviour, 40(4), 396-404.
Bureau of Labor Statistics (2013). American time use survey—2012 results U.S. department of labor. Retrieved January 25, 2014 from http://www.bls.gov/news.release/pdf/atus.pdf
C.E., Chen, K.Y., Freedson, P.S., Buchowski, M.S., Beech, B.M., Pate, R.R., & Troiano, R.P. (2008). Amount of time spent in sedentary behaviors in the United States, 2003-2004. American Journal of Epidemiology, 167 (7), 875-881.
Canadian Task Force on Preventative Health Care (2000). Recommendations on screening for type 2 diabetes in adults. Canadian Medical Association Journal, 184(15), 1687-1696.
Cordain, L., Miller, J. B., Eaton, S. B., Mann, N., Holt, S. H. A., & Speth, J. D. (2000). Plant-animal subsistence ratios and macronutrient energy estimations in worldwide hunter-gatherer diets. The American Journal of Clinical Nutrition, 71, 682-692.
Geragotou, T., Jainandunsing, S., Ozcan, B., Rooij, F. W. M., Kokkinos, A., Tentolouris, N.,Sigbrands, E. J. G. (2016). The relationship of metabolic syndrome traits with beta-cell function and insulin sensitivity by oral minimal model assessment in south Asian and European families residing in he Netherlands. Journal of Diabetes Research, 1-9. doi:10.1155/2016/9286303
Hemoglobin A1c (HbA1c) Test for Diabetes (2017). Retrieved from http://www.webmd.com/diabetes/guide/glycated-hemoglobin-test-hba1c
Hong, J. H., Ku, B. J., & Shong, M. (2015). Response: GD15 is a novel biomarker for impaired fasting glucose. Diabetes and Metabolism Journal, 39(1), 84-86.
Ifland, J. R., Preuss, H. G., Marcus, M. T., Rourke, K. M., Taylor, W. C., Burau, K., … Manso,G. (2009). Refined food addiction: A classic substance use disorder. Medical Hypotheses, 72, 518-526.
Ilich, J. Z., Kelly, O. J., Kim, Y., & Spicer, M. T. (2014). Low-grade chronic inflammation perpetuated by modern diet as a promoter of obesity and osteoporosis. Archives of Industrial Hygiene and Toxicology, 65(2), 139-148.
Iwao, T., Sakai, K., & Ando, E. (2013). Meaning of upper limit of normal range of post-load 1-h plasma glucose level defined by oral glucose tolerance test in Japanese subjects. Journal of Diabetes Investigation, 4(4), 372-375.
Kahn, S. E., Hull, R. L., & Utzshneider, K. M. (2006). Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature, 44(7121), 840-846.
Kenney, W.L., Wilmore, J.H., & Costill, D.L. (2012). Physiology of sport and exercise (5th ed.). Champaign, IL: Human Kinetics.
Lamb, A. (2014). Diabetes and exercise. Clinical Medicine, 14(6), 673-676.
Manscot, A., Oostrom, V., Smit, H. A., Verschuren, W. M. M., & Picavet, H. S. J. (2014). Diagnosis of diabetes mellitus or cardiovascular disease and lifestyle changes — The doetinchem cohort study. Preventative Medicine, 59, 42-46.
Manso,G. (2009). Refined food addiction: A classic substance use disorder. Medical Hypotheses, 72, 518-526.
Mattei, J., Malik, V., Wedick, N. M., Hu, F. B., Spiegelman, D., Willett, W. C., & Campos, H. (2015). Reducing the global burden of type 2 diabetes by improving the quality of staple foods: The global nutrition and epidemic transition initiative. Globalization & Health, 11(1), 1-20.
Matthews, C.E., Chen, K.Y., Freedson, P.S., Buchowski, M.S., Beech, B.M., Pate, R.R., & Troiano, R.P. (2008). Amount of time spent in sedentary behaviors in the United States, 2003-2004. American Journal of Epidemiology, 167 (7), 875-881.
Meckling, K.A., & Sherfey, R. (2007). A randomized trial of a hypocaloric high-protein diet, with and without exercise, on weight loss, fitness, and markers of the metabolic syndrome in overweight and obese women. Applied Physiology, Nutrition & Metabolism, 32(4), 743-752.
Miller, P. E., McKinnon, R. A., Krebs-Smith, S. M., Subar, S. F., Chriqui, J., Kahle, L., & Reedy, J. (2013). Sugar-sweetened beverage consumption in the U.S. American Journal of Preventative Medicine, 34(4), 416-421.
Ochoa, M., Lalles, J. P., Malbert, C. H., & Laillet, D. V. (2015). Dietary sugars: Their detection by the gut-brain axis and their peripheral and central effects in health and diseases. European Journal of Nutrition, 54(1), 1-24.
Paoli, A., Rubini, A., Volek, J. S., & Grimaldi, K. A. (2013). Beyond weight loss: A review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. European Journal of Clinical Nutrition, 67, 789-796.
Reisner, E. G., & Reisner, H. M. (2017). An introduction to human disease: Pathology and pathophysiology correlations (10th ed.). Burlington, MA: Jones & Bartlett Learning.
Russell, N. D. F., & Cooper, M. E. (2015). 50 years forward: Mechanisms of hyperglycemia-driven diabetic complications. Diabetologia, 58(8), 1708-1714.
Zhang, J., Seo, D.C., Kolbe, L., Middletadt, S., & Zhao, W. (2011). Associated trends in sedentary behavior and BMI among Chinese school children and adolescents in seven diverse Chinese provinces. International Journal of Behavioral Medicine, 19 (3), 342-349.
-Michael McIsaac
