
Ascorbic acid (AA), also known as vitamin C, is widely known as an antioxidant and an immune system-enhancing micronutrient. However, AA has other relevant roles to include halting telomere attrition, cell growth, disorganization of chromatin (“packages” and protects DNA in tight bundles), excessive release of inflammatory factors, in addition to prolonging lifespan (Monacelli, Acquarone, Giannotti, Borghi, & Nencioni, 2017). Such qualities, or lack thereof, is also associated with progression of Alzheimer’s disease (AD). As a means of appreciating the role of AA to AD, the following will explore the same.
Monacelli et al. (2017) indicated that epidemiological links between nutrition, a modifiable lifestyle factor, and AD are mounting. Generally, the aging process is characterized by a gradual deterioration of immune system function, also known as immunosenescence (Monacelli et al., 2017). Monacelli et al. (2017) further described immunosenescence as a progressively reduced capacity to respond to pathogens and tolerate self-antigens; such a process inexorably leads to increased vulnerability to infections, autoimmune diseases, cancer, and AD.
AD is characterized by neurodegeneration, and AA has been found to promote biochemical/physiological changes to down-regulate such degeneration (Monacelli et al., 2017). Of particular interest is AA’s capacity to donate electrons to reactive oxygen species (ROS), control neural inflammation, minimize chelation (binding of metals to molecules) of iron/zinc/copper, and suppress beta amyloid plaque production; processes closely related to the pathogenesis of AD (Monacelli et al., 2017).
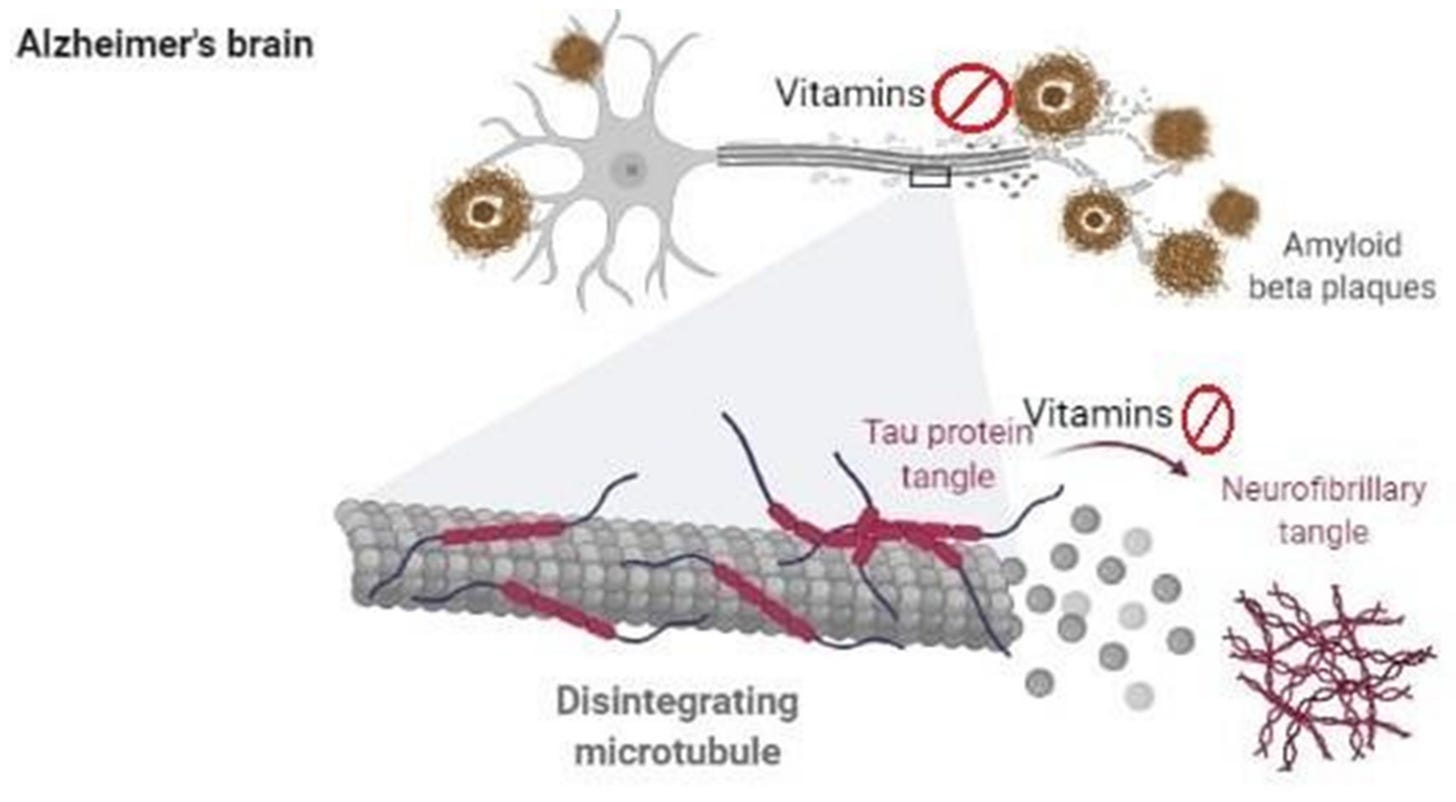
Evidence has suggested that oxidative stress plays a dominant role in the pre-phase of AD, to include mild cognitive impairment (Monacelli et al., 2017). Furthermore, lipid peroxidation also occurs and is identified by the presence of DNA damage (including mitochondrial DNA and nuclear DNA) and oxidized proteins. The development beta-amyloid plaques is another event that manifests during AD; a substance formed from gamma and beta secretase and beta amyloid precursor proteins. Such plaques are neurotoxic and are considered a hallmark of AD (Ono, Watanabe, Watanabe, Haratake, Nakayama, & Saji, 2011). AA is thought to provide neuroprotective effects from the degenerative processes previously outlined (Monacelli et al., 2017).
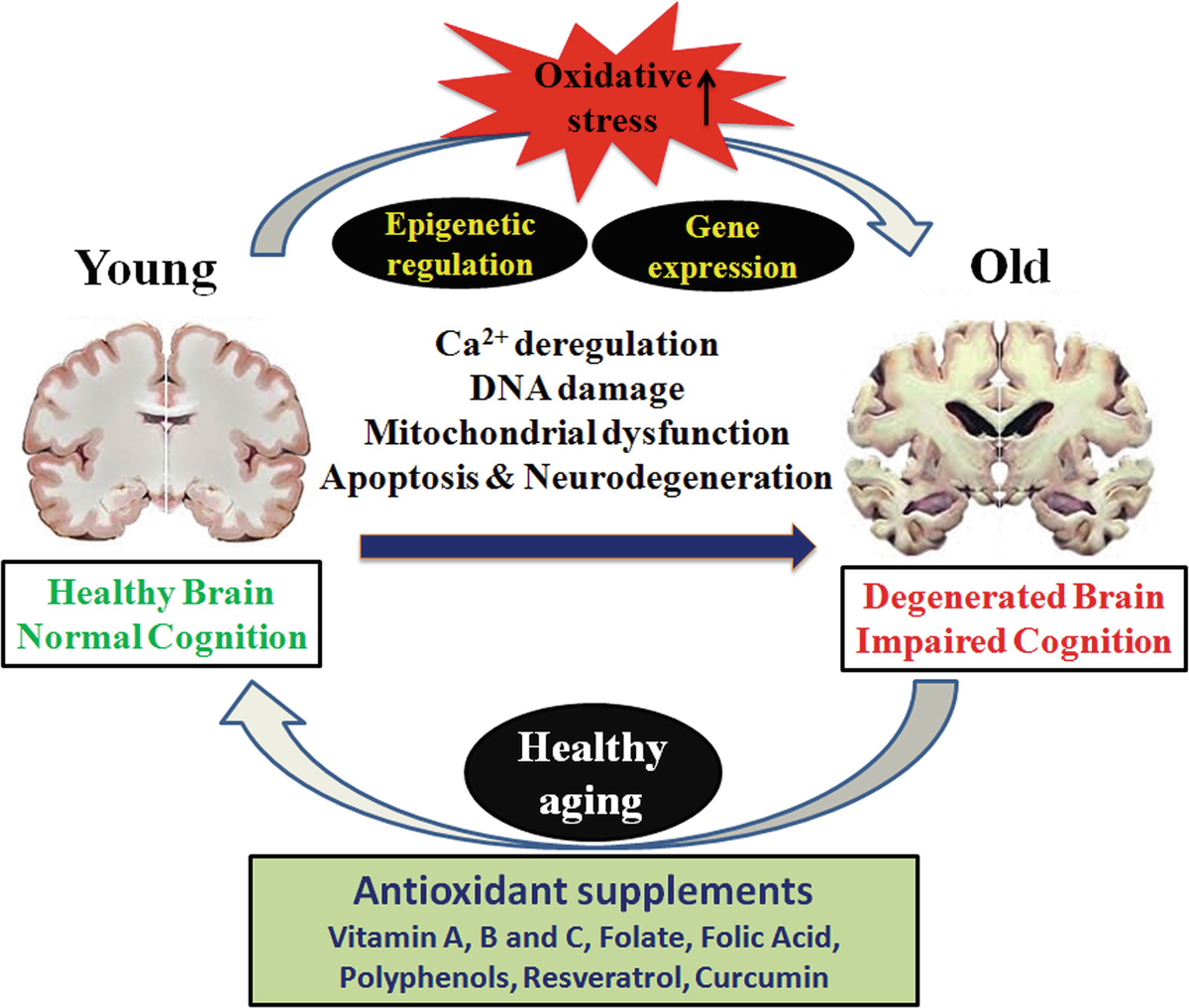
It has been demonstrated that disruption in AA homeostasis (among other nutrients) has been associated in neurodegeneration; AA is released from glial cells and absorbed by neurons as an antioxidant defense mechanism (Monacelli et al., 2017). The researchers stated that such a process was necessary to maintain synaptic function and neuronal metabolism. As such, optimal levels of AA are key to maintain said functions (Monacelli et al., 2017). Furthermore, evidence has suggested that AA in conjunction with another antioxidant, vitamin E, was shown to reduce the risk of developing AD (Monacelli et al., 2017
In conclusion, neurodegenerative processes associated with AD includes low levels of AA. Whether such an occurrence is associative or causative in nature, it might be beneficial and prudent to at least maintain normal levels of AA amongst individuals thought to be at risk or who have AD. Such an approach is likely to be part of a larger intervention in the treatment and prevention of AD.
References
Monacelli, F., Acquarone, E., Giannotti, C., Borghi, C., & Nencioni, A. (2017). Vitamin C, aging and Alzheimer’s disease. Nutrients, 9(7), 670.
Ono, M., Watanabe, H., Watanabe, R., Haratake, M., Nakayama, M., & Saji, H. (2011). Diphenylpropynone derivatives as probes for imaging b-amyloid plaques in Alzheimer’s disease. Bioorganic & Medicinal Chemistry Letters, 21(1), 117-120.
-Michael McIsaac
